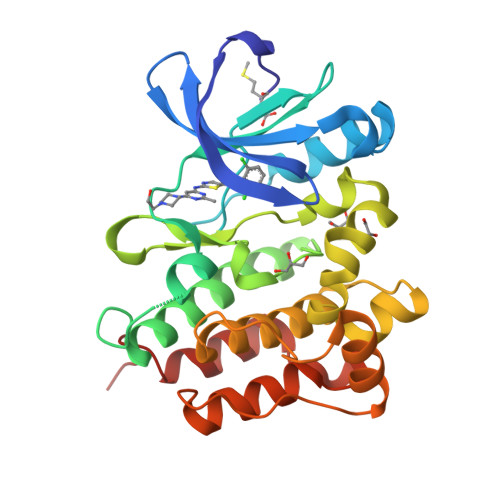
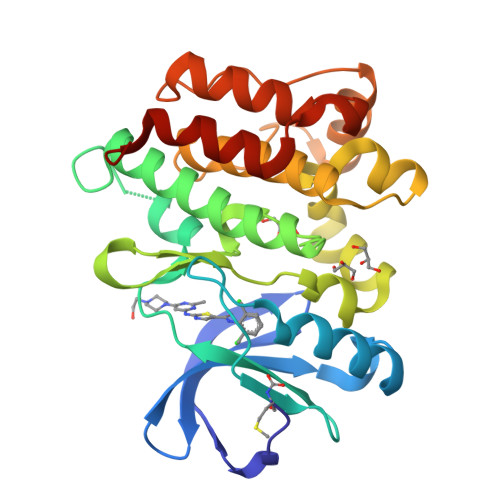
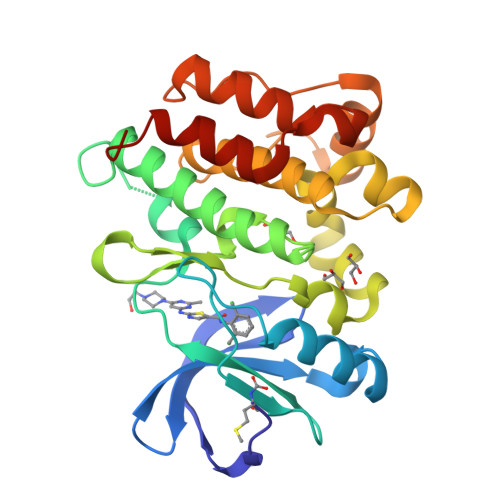
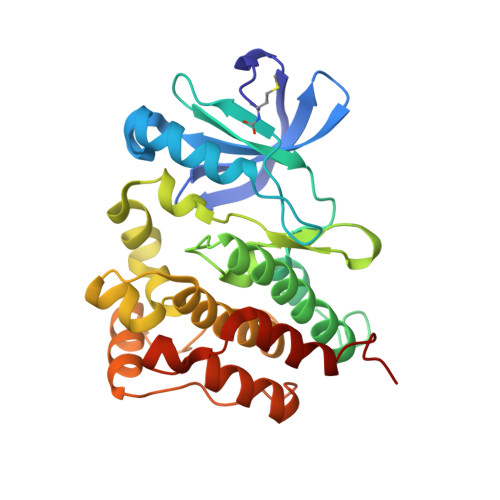
Co-crystal structures of PTK6: With Dasatinib at 2.24 angstrom , with novel imidazo[1,2-a]pyrazin-8-amine derivative inhibitor at 1.70 angstrom resolution
Thakur, M.K., Birudukota, S., Swaminathan, S., Battula, S.K., Vadivelu, S., Tyagi, R., Gosu, R.(2017) Biochem Biophys Res Commun 482: 1289-1295
- PubMed: 27993680
- DOI: https://doi.org/10.1016/j.bbrc.2016.12.030
- Primary Citation of Related Structures:
5DA3, 5H2U - PubMed Abstract:
Human Protein tyrosine kinase 6 (PTK6)(EC:2.7.10.2), also known as the breast tumor kinase (BRK), is an intracellular non-receptor Src-related tyrosine kinase expressed five-fold or more in human breast tumors and breast cancer cell lines but its expression being low or completely absent from normal mammary gland. There is a recent interest in targeting PTK6-positive breast cancer by developing small molecule inhibitor against PTK6. Novel imidazo[1,2-a]pyrazin-8-amines (IPA) derivative compounds and FDA approved drug, Dasatinib are reported to inhibit PTK6 kinase activity with IC 50 in nM range. To understand binding mode of these compounds and key interactions that drive the potency against PTK6, one of the IPA compounds and Dasatinib were chosen to study through X-ray crystallography. The recombinant PTK6 kinase domain was purified and co-crystallized at room temperature by the sitting-drop vapor diffusion method, collected X-ray diffraction data at in-house and resolved co-crystal structure of PTK6-KD with Dasatinib at 2.24 Å and with IPA compound at 1.70 Å resolution. Both these structures are in DFG-in & αC-helix-out conformation with unambiguous electron density for Dasatinib or IPA compound bound at the ATP-binding pocket. Relative difference in potency between Dasatinib and IPA compound is delineated through the additional interactions derived from the occupation of additional pocket by Dasatinib at gatekeeper area. Refined crystallographic coordinates for the kinase domain of PTK6 in complex with IPA compound and Dasatinib have been submitted to Protein Data Bank under the accession number 5DA3 and 5H2U respectively.
Organizational Affiliation:
Department of Biochemistry, University of Mysore, Mysore, 570005, India; Department of Structural Biology, Jubilant Biosys Ltd, Bangalore, 560022, India.