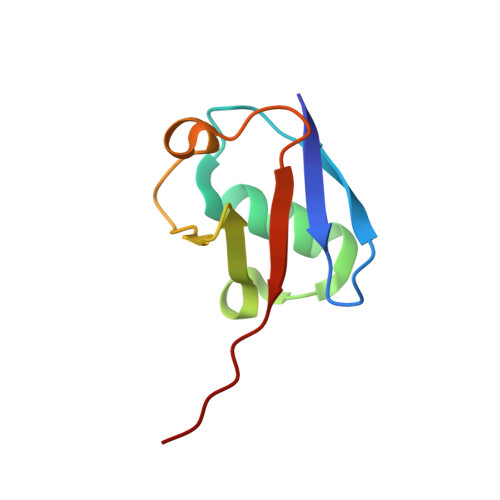
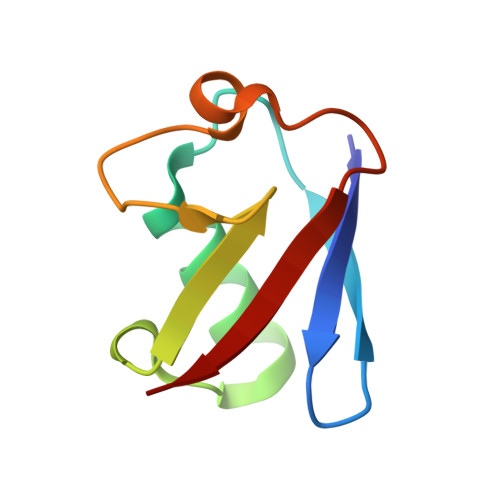
Structural basis for specific cleavage of Lys6-linked polyubiquitin chains by USP30
Sato, Y., Okatsu, K., Saeki, Y., Yamano, K., Matsuda, N., Kaiho, A., Yamagata, A., Goto-Ito, S., Ishikawa, M., Hashimoto, Y., Tanaka, K., Fukai, S.(2017) Nat Struct Mol Biol 24: 911-919
- PubMed: 28945247
- DOI: https://doi.org/10.1038/nsmb.3469
- Primary Citation of Related Structures:
5GVI - PubMed Abstract:
Parkin ubiquitin (Ub) ligase (also known as PARK2) ubiquitinates damaged mitochondria for their clearance and quality control. USP30 deubiquitinase opposes parkin-mediated Ub-chain formation on mitochondria by preferentially cleaving Lys6-linked Ub chains. Here, we report the crystal structure of zebrafish USP30 in complex with a Lys6-linked diubiquitin (diUb or Ub 2 ) at 1.87-Å resolution. The distal Ub-recognition mechanism of USP30 is similar to those of other USP family members, whereas Phe4 and Thr12 of the proximal Ub are recognized by a USP30-specific surface. Structure-based mutagenesis showed that the interface with the proximal Ub is critical for the specific cleavage of Lys6-linked Ub chains, together with the noncanonical catalytic triad composed of Cys-His-Ser. The structural findings presented here reveal a mechanism for Lys6-linkage-specific deubiquitination.
Organizational Affiliation:
Structural Life Science Division, Synchrotron Radiation Research Organization, The University of Tokyo, Tokyo, Japan.