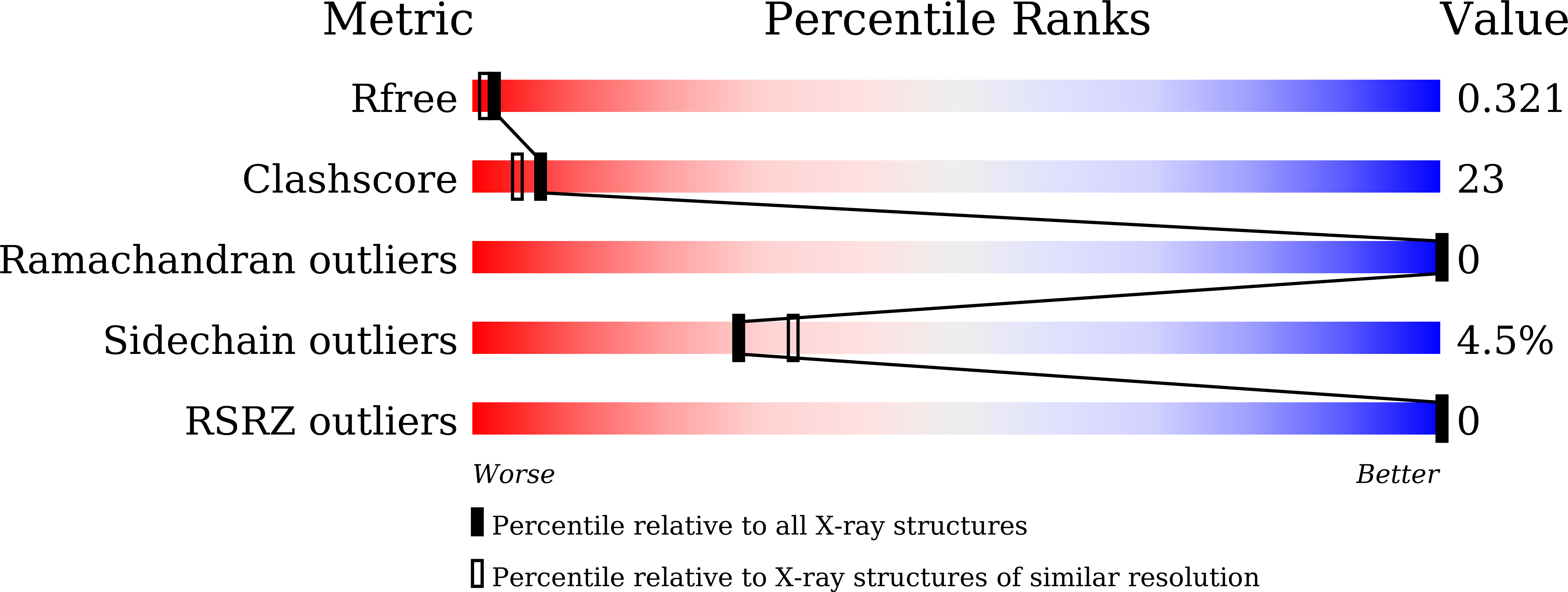
Monomer/Oligomer Quasi-Racemic Protein Crystallography
Gao, S., Pan, M., Zheng, Y., Huang, Y., Zheng, Q., Sun, D., Lu, L., Tan, X., Tan, X., Lan, H., Wang, J., Wang, T., Wang, J., Liu, L.(2016) J Am Chem Soc 138: 14497-14502
- PubMed: 27768314
- DOI: https://doi.org/10.1021/jacs.6b09545
- Primary Citation of Related Structures:
5GO7, 5GO8, 5GOB, 5GOC, 5GOD, 5GOG, 5GOH, 5GOI, 5GOJ, 5GOK - PubMed Abstract:
Racemic or quasi-racemic crystallography recently emerges as a useful technology for solution of the crystal structures of biomacromolecules. It remains unclear to what extent the biomacromolecules of opposite handedness can differ from each other in racemic or quasi-racemic crystallography. Here we report a finding that monomeric d-ubiquitin (Ub) has propensity to cocrystallize with different dimers, trimers, and even a tetramer of l-Ub. In these cocrystals the unconnected monomeric d-Ubs can self-assemble to form pseudomirror images of different oligomers of l-Ub. This monomer/oligomer cocrystallization phenomenon expands the concept of racemic crystallography. Using the monomer/oligomer cocrystallization technology we obtained, for the first time the X-ray structures of linear M1-linked tri- and tetra-Ubs and a K11/K63-branched tri-Ub.
Organizational Affiliation:
Tsinghua-Peking Center for Life Sciences, Ministry of Education Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology, Department of Chemistry and ‡State Key Laboratory of Biomembrane and Membrane Biotechnology, Center for Structural Biology, School of Life Sciences, Tsinghua University , Beijing 100084, China.