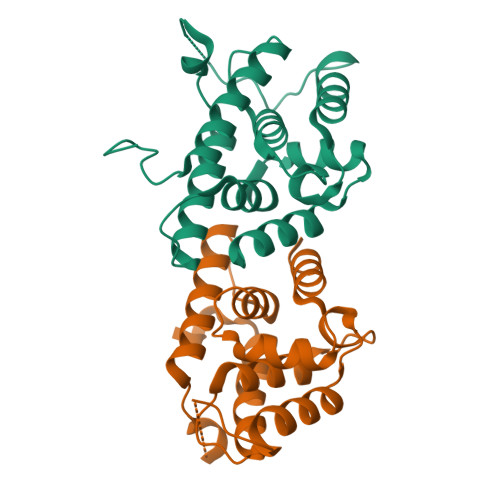
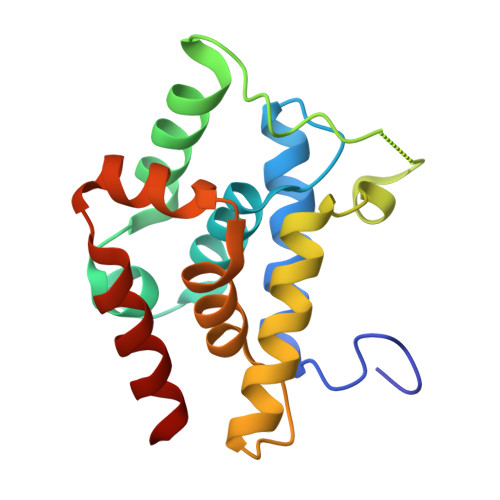
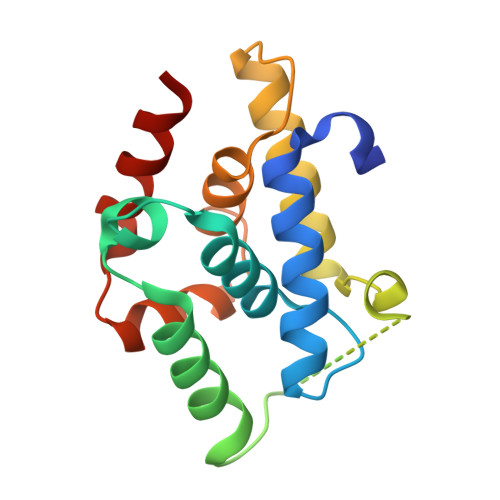
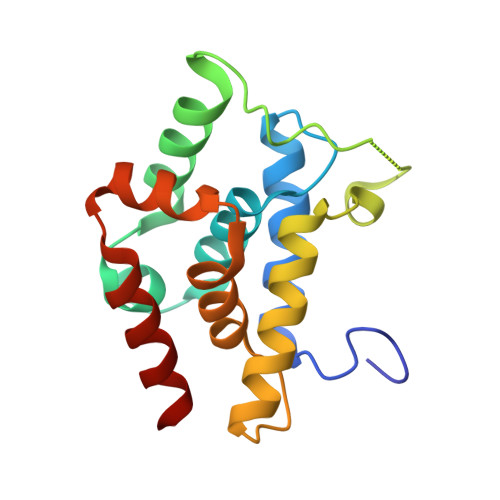
Crystal structures reveal N-terminal Domain of Arabidopsis thaliana ClpD to be highly divergent from that of ClpC1.
Mohapatra, C., Kumar Jagdev, M., Vasudevan, D.(2017) Sci Rep 7: 44366-44366
- PubMed: 28287170
- DOI: https://doi.org/10.1038/srep44366
- Primary Citation of Related Structures:
5GKM, 5GUI - PubMed Abstract:
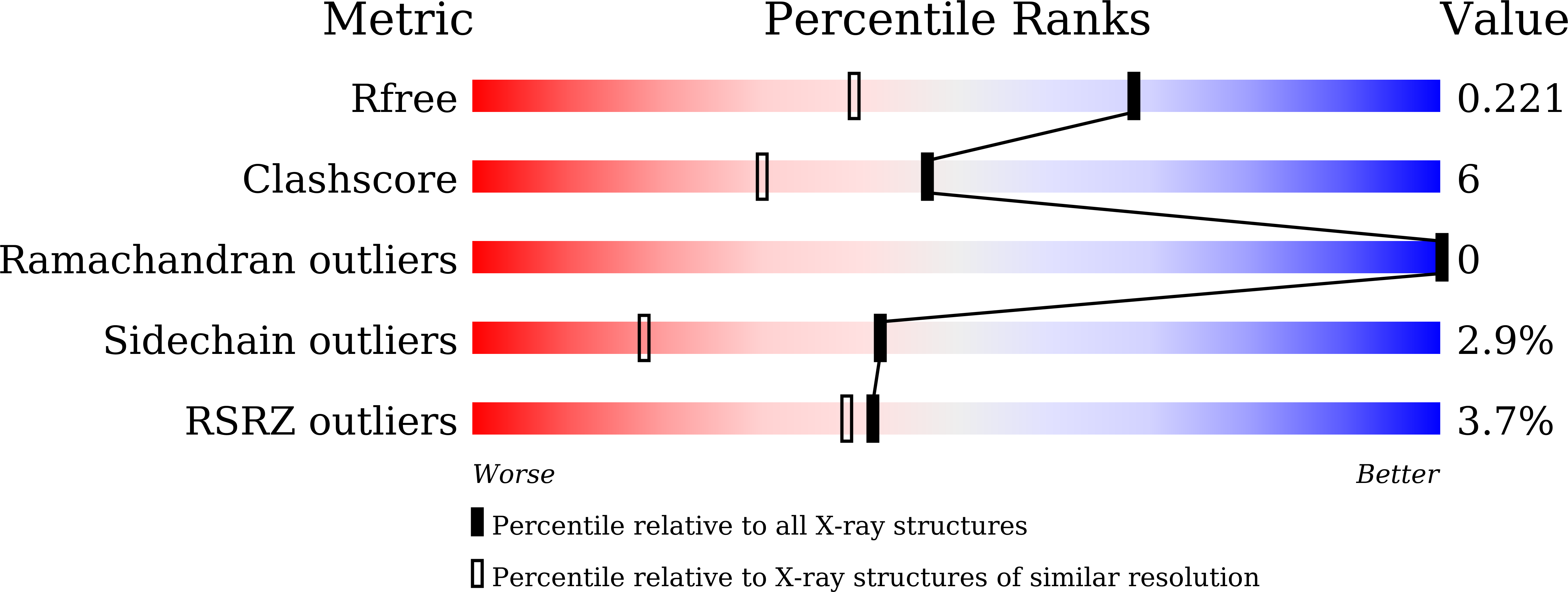
The caseinolytic protease machinery associated chaperone protein ClpC is known to be present in bacteria, plants and other eukaryotes, whereas ClpD is unique to plants. Plant ClpC and ClpD proteins get localized into chloroplast stroma. Herein, we report high resolution crystal structures of the N-terminal domain of Arabidopsis thaliana ClpC1 and ClpD. Surprisingly, AtClpD, but not AtClpC1, deviates from the typical N-terminal repeat domain organization of known Clp chaperones and have only seven α-helices, instead of eight. In addition, the loop connecting the two halves of AtClpD NTD is longer and covers the region which in case of AtClpC1 is thought to contribute to adaptor protein interaction. Taken together, the N-terminal domain of AtClpD has a divergent structural organization compared to any known Clp chaperones which hints towards its specific role during plant stress conditions, as opposed to that in the maintenance of chloroplastic homeostasis by AtClpC1. Conservation of residues in the NTD that are responsible for the binding of the cyclic peptide activator - Cyclomarin A, as reported for mycobacterial ClpC1 suggests that the peptide could be used as an activator to both AtClpC1 and AtClpD, which could be useful in their detailed in vitro functional characterization.
Organizational Affiliation:
Institute of Life Sciences, Bhubaneswar - 751023, Odisha State, India.