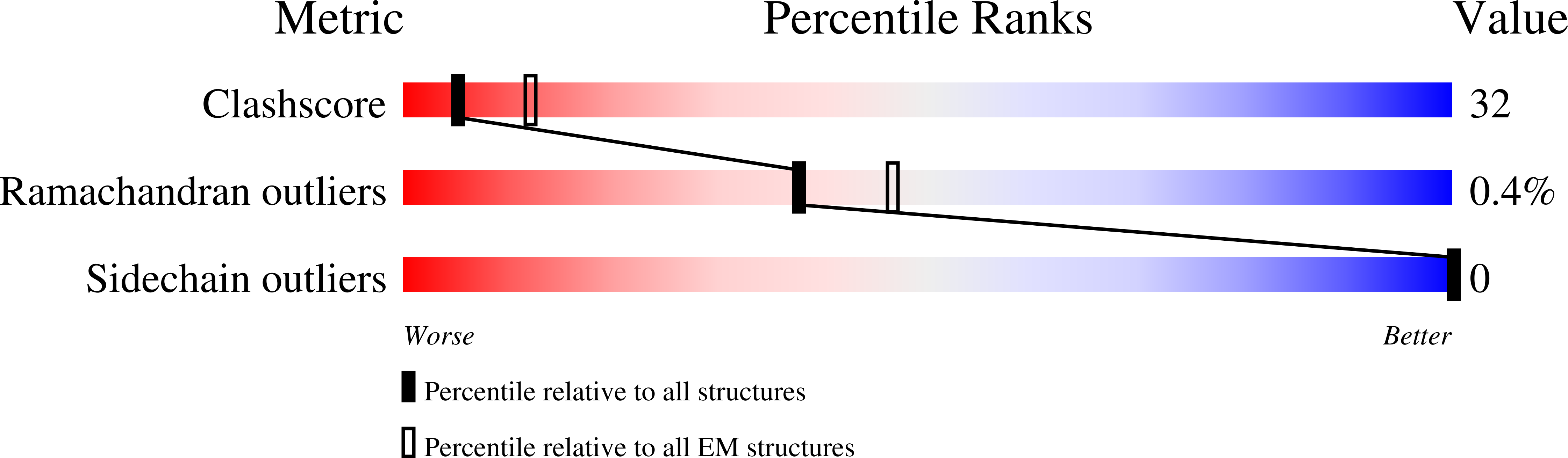
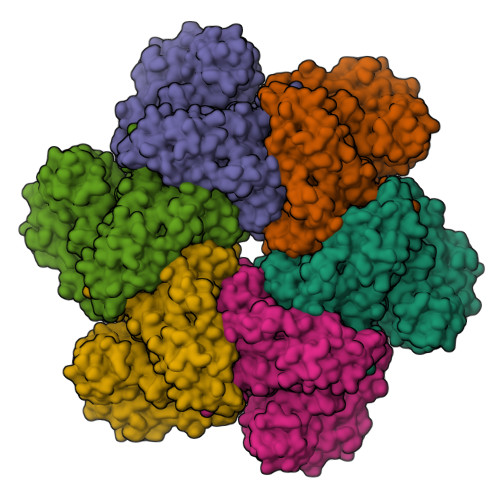
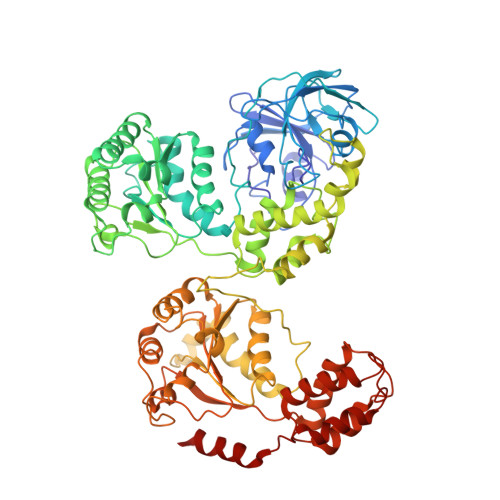
Unfolding the Mechanism of the Aaa+ Unfoldase Vat by a Combined Cryo-Em, Solution NMR Study.
Huang, R., Ripstein, Z.A., Augustyniak, R., Lazniewski, M., Ginalski, K., Kay, L.E., Rubinstein, J.L.(2016) Proc Natl Acad Sci U S A 113: E4190
- PubMed: 27402735
- DOI: https://doi.org/10.1073/pnas.1603980113
- Primary Citation of Related Structures:
5G4F, 5G4G - PubMed Abstract:
The AAA+ (ATPases associated with a variety of cellular activities) enzymes play critical roles in a variety of homeostatic processes in all kingdoms of life. Valosin-containing protein-like ATPase of Thermoplasma acidophilum (VAT), the archaeal homolog of the ubiquitous AAA+ protein Cdc48/p97, functions in concert with the 20S proteasome by unfolding substrates and passing them on for degradation. Here, we present electron cryomicroscopy (cryo-EM) maps showing that VAT undergoes large conformational rearrangements during its ATP hydrolysis cycle that differ dramatically from the conformational states observed for Cdc48/p97. We validate key features of the model with biochemical and solution methyl-transverse relaxation optimized spectroscopY (TROSY) NMR experiments and suggest a mechanism for coupling the energy of nucleotide hydrolysis to substrate unfolding. These findings illustrate the unique complementarity between cryo-EM and solution NMR for studies of molecular machines, showing that the structural properties of VAT, as well as the population distributions of conformers, are similar in the frozen specimens used for cryo-EM and in the solution phase where NMR spectra are recorded.
Organizational Affiliation:
Department of Molecular Genetics, University of Toronto, Toronto, ON, Canada M5S 1A8; Department of Chemistry, University of Toronto, Toronto, ON, Canada M5S 3H6; Department of Biochemistry, University of Toronto, Toronto, ON, Canada M5S 1A8;