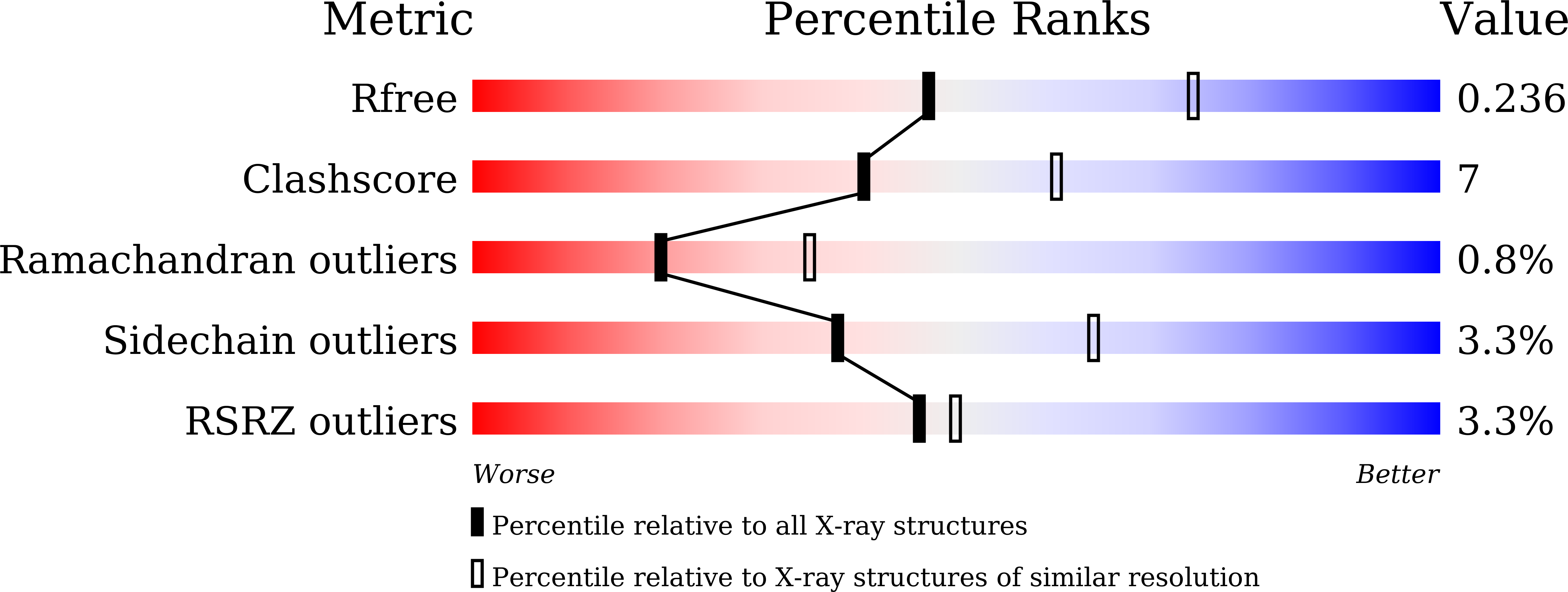
Structural elucidation of the NADP(H) phosphatase activity of staphylococcal dual-specific IMPase/NADP(H) phosphatase
Bhattacharyya, S., Dutta, A., Dutta, D., Ghosh, A.K., Das, A.K.(2016) Acta Crystallogr D Struct Biol 72: 281-290
- PubMed: 26894675
- DOI: https://doi.org/10.1107/S2059798316000620
- Primary Citation of Related Structures:
5EYG, 5EYH, 5F24 - PubMed Abstract:
NADP(H)/NAD(H) homeostasis has long been identified to play a pivotal role in the mitigation of reactive oxygen stress (ROS) in the intracellular milieu and is therefore critical for the progression and pathogenesis of many diseases. NAD(H) kinases and NADP(H) phosphatases are two key players in this pathway. Despite structural evidence demonstrating the existence and mode of action of NAD(H) kinases, the specific annotation and the mode of action of NADP(H) phosphatases remains obscure. Here, structural evidence supporting the alternative role of inositol monophosphatase (IMPase) as an NADP(H) phosphatase is reported. Crystal structures of staphylococcal dual-specific IMPase/NADP(H) phosphatase (SaIMPase-I) in complex with the substrates D-myo-inositol-1-phosphate and NADP(+) have been solved. The structure of the SaIMPase-I-Ca(2+)-NADP(+) ternary complex reveals the catalytic mode of action of NADP(H) phosphatase. Moreover, structures of SaIMPase-I-Ca(2+)-substrate complexes have reinforced the earlier proposal that the length of the active-site-distant helix α4 and its preceding loop are the predisposing factors for the promiscuous substrate specificity of SaIMPase-I. Altogether, the evidence presented suggests that IMPase-family enzymes with a shorter α4 helix could be potential candidates for previously unreported NADP(H) phosphatase activity.
Organizational Affiliation:
Department of Biotechnology, Indian Institute of Technology Kharagpur, Kharagpur 721 302, India.