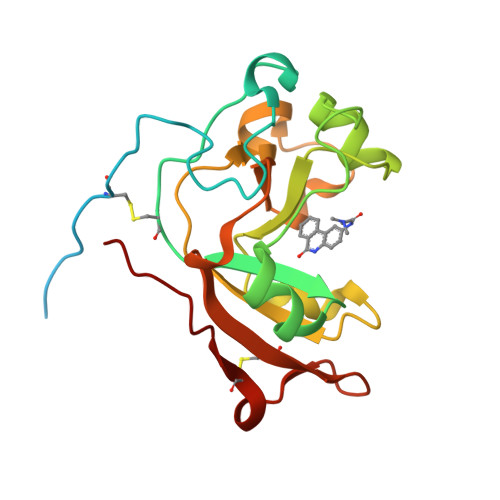
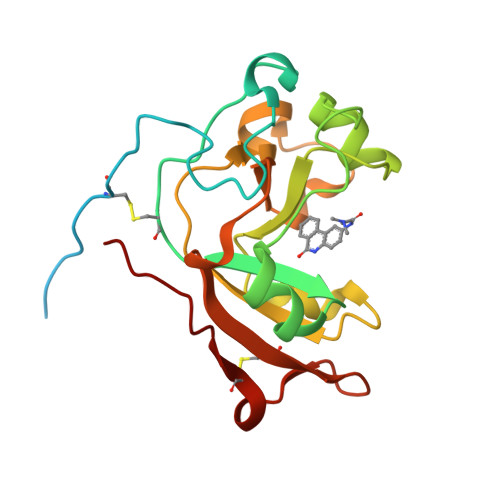
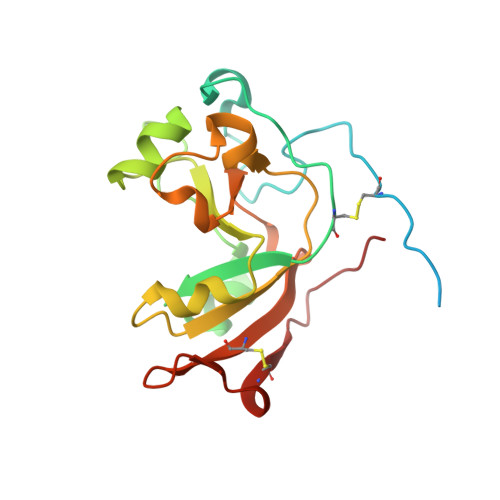
Scabin, a Novel DNA-acting ADP-ribosyltransferase from Streptomyces scabies.
Lyons, B., Ravulapalli, R., Lanoue, J., Lugo, M.R., Dutta, D., Carlin, S., Merrill, A.R.(2016) J Biological Chem 291: 11198-11215
- PubMed: 27002155
- DOI: https://doi.org/10.1074/jbc.M115.707653
- Primary Citation of Related Structures:
5DAZ, 5EWK, 5EWY - PubMed Abstract:
A bioinformatics strategy was used to identify Scabin, a novel DNA-targeting enzyme from the plant pathogen 87.22 strain of Streptomyces scabies Scabin shares nearly 40% sequence identity with the Pierisin family of mono-ADP-ribosyltransferase toxins. Scabin was purified to homogeneity as a 22-kDa single-domain enzyme and was shown to possess high NAD(+)-glycohydrolase (Km (NAD) = 68 ± 3 μm; kcat = 94 ± 2 min(-1)) activity with an RSQXE motif; it was also shown to target deoxyguanosine and showed sigmoidal enzyme kinetics (K0.5(deoxyguanosine) = 302 ± 12 μm; kcat = 14 min(-1)). Mass spectrometry analysis revealed that Scabin labels the exocyclic amino group on guanine bases in either single-stranded or double-stranded DNA. Several small molecule inhibitors were identified, and the most potent compounds were found to inhibit the enzyme activity with Ki values ranging from 3 to 24 μm PJ34, a well known inhibitor of poly-ADP-ribosyltransferases, was shown to be the most potent inhibitor of Scabin. Scabin was crystallized, representing the first structure of a DNA-targeting mono-ADP-ribosyltransferase enzyme; the structures of the apo-form (1.45 Å) and with two inhibitors (P6-E, 1.4 Å; PJ34, 1.6 Å) were solved. These x-ray structures are also the first high resolution structures of the Pierisin subgroup of the mono-ADP-ribosyltransferase toxin family. A model of Scabin with its DNA substrate is also proposed.
Organizational Affiliation:
From the Department of Molecular and Cellular Biology, University of Guelph, Guelph, Ontario N1G 2W1, Canada and.