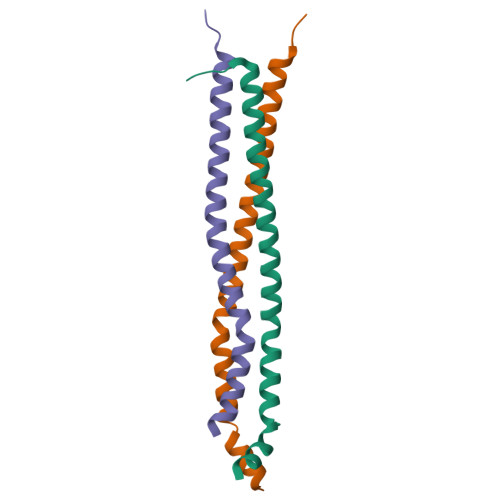
Coiled-Coil Domains of SUN Proteins as Intrinsic Dynamic Regulators
Nie, S., Ke, H., Gao, F., Ren, J., Wang, M., Huo, L., Gong, W., Feng, W.(2016) Structure 24: 80-91
- PubMed: 26688217
- DOI: https://doi.org/10.1016/j.str.2015.10.024
- Primary Citation of Related Structures:
5ED8, 5ED9 - PubMed Abstract:
SUN proteins are the core components of LINC complexes that span across the nuclear envelope for nuclear positioning and migration. SUN proteins contain at least one predicted coiled-coil domain preceding the SUN domain. Here, we found that the two coiled-coil domains (CC1 and CC2) of SUN2 exhibit distinct oligomeric states. CC2 is a monomer in solution. The structure of the CC2-SUN monomer revealed that CC2 unexpectedly folds as a three-helix bundle that interacts with the SUN domain and locks it in an inactive conformation. In contrast, CC1 is a trimer. The structure of the CC1 trimer demonstrated that CC1 is an imperfect coiled coil for the trimerization and activation of the SUN domain. Modulations of CC1 and CC2 dictate different oligomeric states of CC1-CC2-SUN, which is essential for LINC complex formation. Thus, the two coiled-coil domains of SUN2 act as the intrinsic dynamic regulators for controlling the SUN domain activity.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Beijing 100101, China.