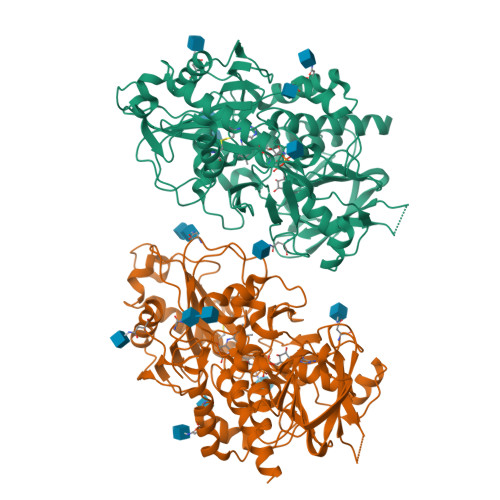
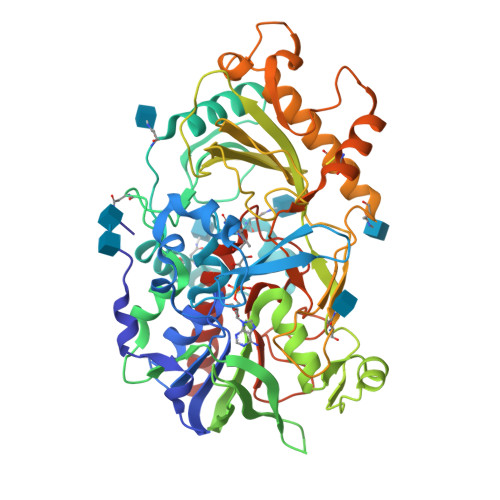
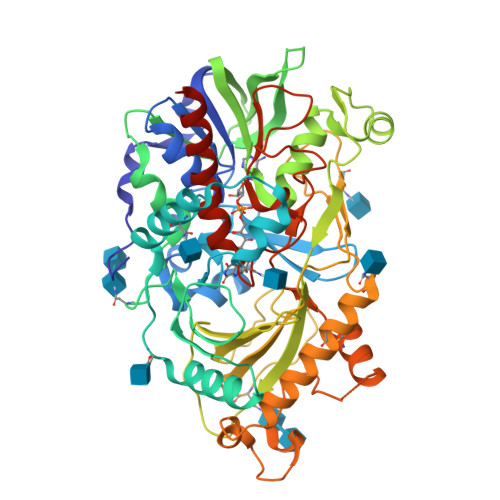
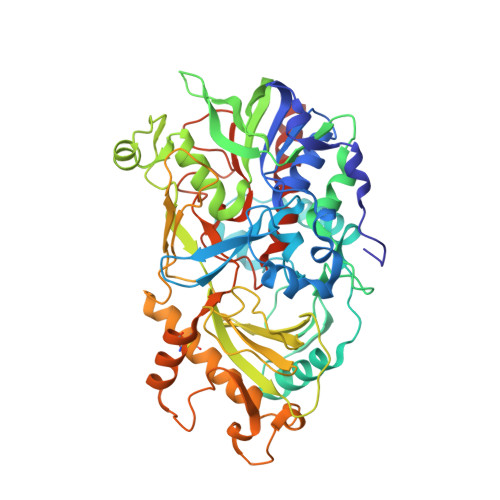
Structures of almond hydroxynitrile lyase isoenzyme 5 provide a rationale for the lack of oxidoreductase activity in flavin dependent HNLs.
Pavkov-Keller, T., Bakhuis, J., Steinkellner, G., Jolink, F., Keijmel, E., Birner-Gruenberger, R., Gruber, K.(2016) J Biotechnol 235: 24-31
- PubMed: 27067080
- DOI: https://doi.org/10.1016/j.jbiotec.2016.04.013
- Primary Citation of Related Structures:
5EB4, 5EB5 - PubMed Abstract:
Hydroxynitrile lyases (HNLs) catalyze the asymmetric addition of HCN to aldehydes producing enantiomerically pure cyanohydrins. These enzymes can be heterologously expressed in large quantities making them interesting candidates for industrial applications. The HNLs from Rosaceae evolved from flavin dependent dehydrogenase/oxidase structures. Here we report the high resolution X-ray structure of the highly glycosylated Prunus amygdalus HNL isoenzyme5 (PaHNL5 V317A) expressed in Aspergillus niger and its complex with benzyl alcohol. A comparison with the structure of isoenzyme PaHNL1 indicates a higher accessibility to the active site and a larger cavity for PaHNL5. Additionally, the PaHNL5 complex structure with benzyl alcohol was compared with the structurally related aryl-alcohol oxidase (AAO). Even though both enzymes contain an FAD-cofactor and histidine residues at crucial positions in the active site, PaHNL5 lacks the oxidoreductase activity. The structures indicate that in PaHNLs benzyl alcohol is bound too far away from the FAD cofactor in order to be oxidized.
Organizational Affiliation:
ACIB GmbH, Petersgasse 14, 8010 Graz, Austria; Institute of Molecular Biosciences, University of Graz, Humboldtstraße 50/3, 8010 Graz, Austria.