Chemical Probes Allow Structural Insight into the Condensation Reaction of Nonribosomal Peptide Synthetases.
Bloudoff, K., Alonzo, D.A., Schmeing, T.M.(2016) Cell Chem Biol 23: 331-339
- PubMed: 26991102
- DOI: https://doi.org/10.1016/j.chembiol.2016.02.012
- Primary Citation of Related Structures:
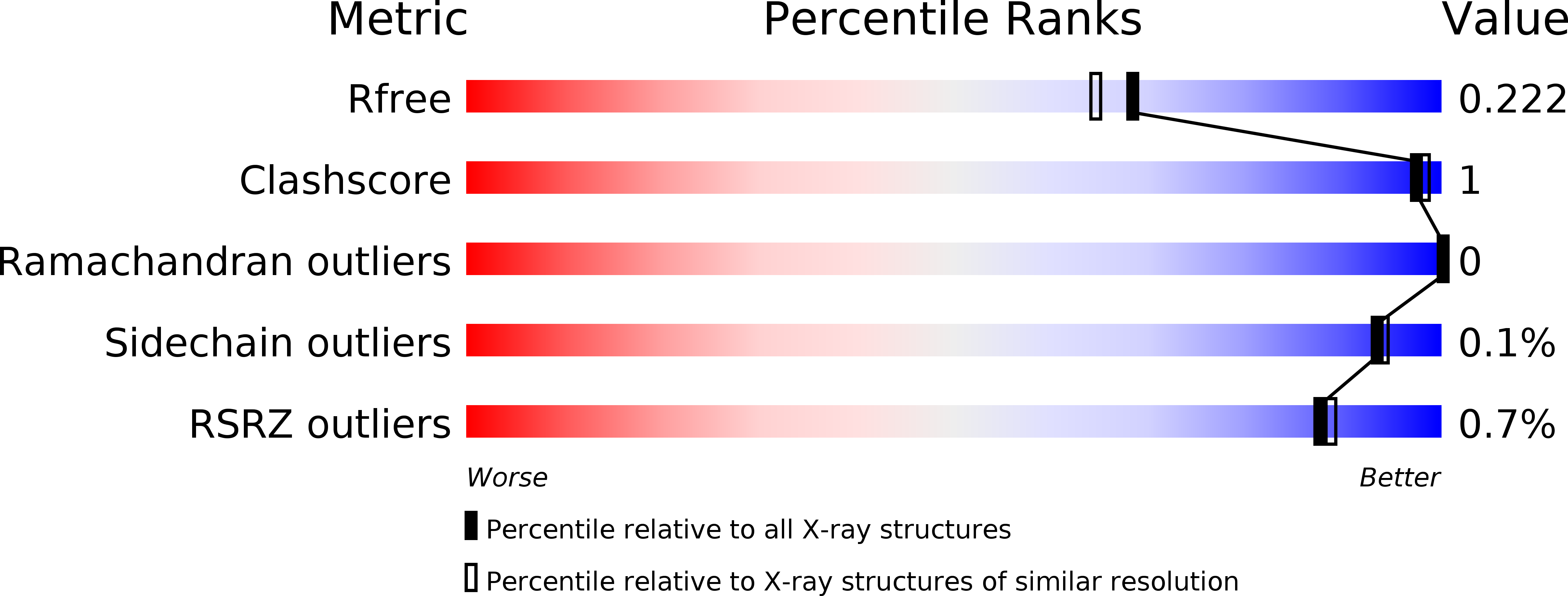
5DU9, 5DUA - PubMed Abstract:
Nonribosomal peptide synthetases (NRPSs) synthesize a vast variety of small molecules, including antibiotics, antitumors, and immunosuppressants. The NRPS condensation (C) domain catalyzes amide bond formation, the central chemical step in nonribosomal peptide synthesis. The catalytic mechanism and substrate determinants of the reaction are under debate. We developed chemical probes to structurally study the NRPS condensation reaction. These substrate analogs become covalently tethered to a cysteine introduced near the active site, to mimic covalent substrate delivery by carrier domains. They are competent substrates in the condensation reaction and behave similarly to native substrates. Co-crystal structures show C domain-substrate interactions, and suggest that the catalytic histidine's principle role is to position the α-amino group for nucleophilic attack. Structural insight provided by these co-complexes also allowed us to alter the substrate specificity profile of the reaction with a single point mutation.
Organizational Affiliation:
Department of Biochemistry, McGill University, 3649 Promenade Sir William Osler, Francesco Bellini Life Sciences Building, Room 465, Montréal, QC H3G 0B1, Canada.