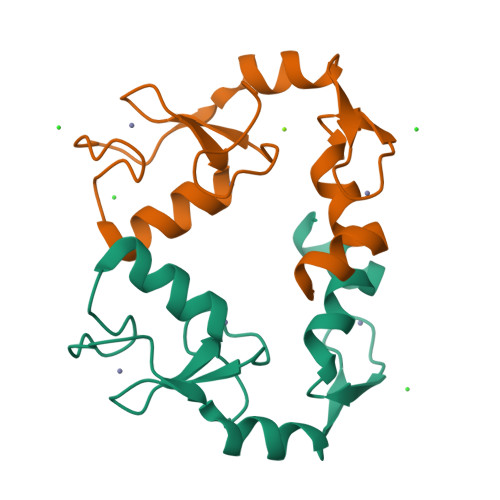
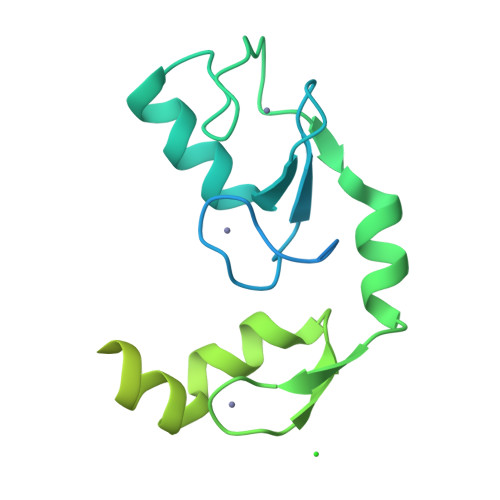
A C2HC zinc finger is essential for the RING-E2 interaction of the ubiquitin ligase RNF125.
Bijlmakers, M.J., Teixeira, J.M., Boer, R., Mayzel, M., Puig-Sarries, P., Karlsson, G., Coll, M., Pons, M., Crosas, B.(2016) Sci Rep 6: 29232-29232
- PubMed: 27411375
- DOI: https://doi.org/10.1038/srep29232
- Primary Citation of Related Structures:
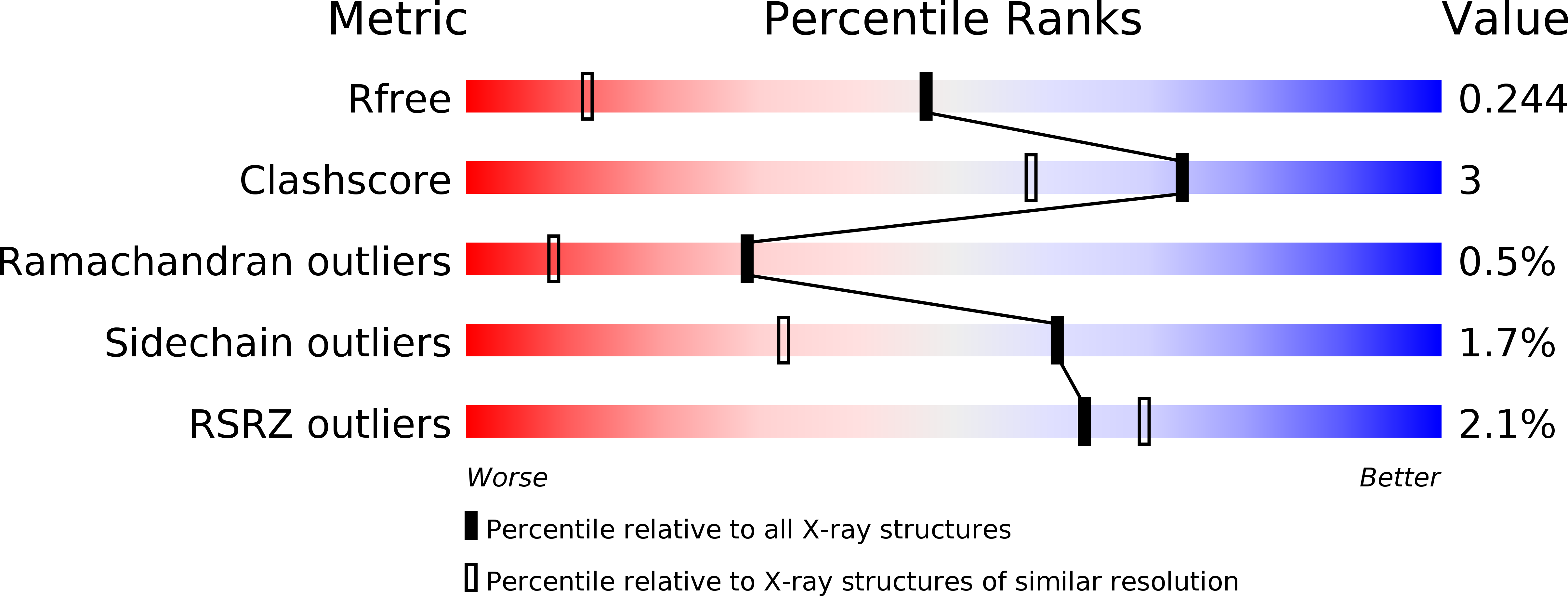
5DKA - PubMed Abstract:
The activity of RING ubiquitin ligases (E3s) depends on an interaction between the RING domain and ubiquitin conjugating enzymes (E2), but posttranslational events or additional structural elements, yet largely undefined, are frequently required to enhance or regulate activity. Here, we show for the ubiquitin ligase RNF125 that, in addition to the RING domain, a C2HC Zn finger (ZnF) is crucial for activity, and a short linker sequence (Li2(120-128)) enhances activity. The contribution of these regions was first shown with truncated proteins, and the essential role of the ZnF was confirmed with mutations at the Zn chelating Cys residues. Using NMR, we established that the C2HC ZnF/Li2(120-128) region is crucial for binding of the RING domain to the E2 UbcH5a. The partial X-ray structure of RNF125 revealed the presence of extensive intramolecular interactions between the RING and C2HC ZnF. A mutation at one of the contact residues in the C2HC ZnF, a highly conserved M112, resulted in the loss of ubiquitin ligase activity. Thus, we identified the structural basis for an essential role of the C2HC ZnF and conclude that this domain stabilizes the RING domain, and is therefore required for binding of RNF125 to an E2.
Organizational Affiliation:
Institut de Biologia Molecular de Barcelona, CSIC, Parc Cientific de Barcelona, 08028 Barcelona, Spain.