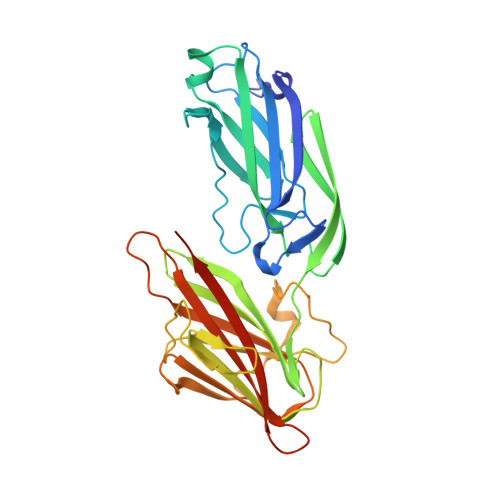
Crystal structures of Bbp from Staphylococcus aureus reveal the ligand binding mechanism with Fibrinogen alpha
Zhang, X.Y., Wu, M., Zhuo, W., Gu, J.K., Zhang, S.S., Ge, J.P., Yang, M.J.(2015) Protein Cell 6: 757-766
- PubMed: 26349459
- DOI: https://doi.org/10.1007/s13238-015-0205-x
- Primary Citation of Related Structures:
5CF3, 5CFA - PubMed Abstract:
Bone sialoprotein-binding protein (Bbp), a MSCRAMMs (Microbial Surface Components Recognizing Adhesive Matrix Molecules) family protein expressed on the surface of Staphylococcus aureus (S. aureus), mediates adherence to fibrinogen α (Fg α), a component in the extracellular matrix of the host cell and is important for infection and pathogenesis. In this study, we solved the crystal structures of apo-Bbp(273-598) and Bbp(273-598)-Fg α(561-575) complex at a resolution of 2.03 Å and 1.45 Å, respectively. Apo-Bbp(273-598) contained the ligand binding region N2 and N3 domains, both of which followed a DE variant IgG fold characterized by an additional D1 strand in N2 domain and D1' and D2' strands in N3 domain. The peptide mapped to the Fg α(561-575) bond to Bbp(273-598) on the open groove between the N2 and N3 domains. Strikingly, the disordered C-terminus in the apo-form reorganized into a highly-ordered loop and a β-strand G'' covering the ligand upon ligand binding. Bbp(Ala298-Gly301) in the N2 domain of the Bbp(273-598)-Fg α(561-575) complex, which is a loop in the apo-form, formed a short α-helix to interact tightly with the peptide. In addition, Bbp(Ser547-Gln561) in the N3 domain moved toward the binding groove to make contact directly with the peptide, while Bbp(Asp338-Gly355) and Bbp(Thr365-Tyr387) in N2 domain shifted their configurations to stabilize the reorganized C-terminus mainly through strong hydrogen bonds. Altogether, our results revealed the molecular basis for Bbp-ligand interaction and advanced our understanding of S. aureus infection process.
Organizational Affiliation:
Key Laboratory for Protein Sciences of Ministry of Education, Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing, 100084, China.