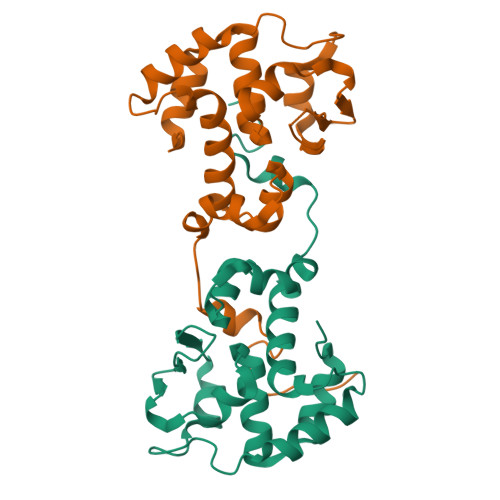
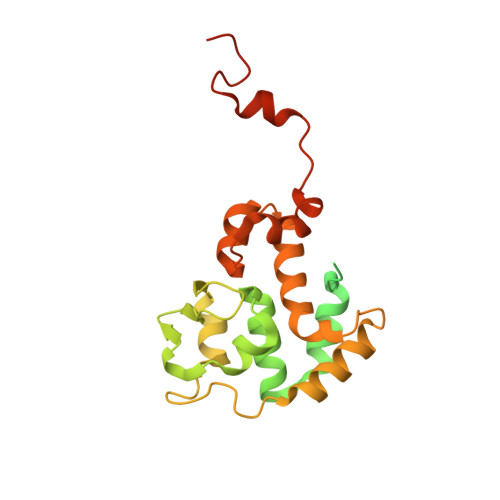
Crystal structure of the bacteriophage P2 integrase catalytic domain.
Skaar, K., Claesson, M., Odegrip, R., Hogbom, M., Haggard-Ljungquist, E., Stenmark, P.(2015) FEBS Lett 589: 3556-3563
- PubMed: 26453836
- DOI: https://doi.org/10.1016/j.febslet.2015.09.026
- Primary Citation of Related Structures:
5C6K, 5DOR - PubMed Abstract:
Bacteriophage P2 is a temperate phage capable of integrating its DNA into the host genome by site-specific recombination upon lysogenization. Integration and excision of the phage genome requires P2 integrase, which performs recognition, cleavage and joining of DNA during these processes. This work presents the high-resolution crystal structure of the catalytic domain of P2 integrase, and analysis of the structure-function relationship of several previously identified non-functional P2 integrase mutants. The DNA binding area is characterized by a large positively charged patch, harboring key residues. The structure reveals potential for large dimer flexibility, likely essential for rearrangement of DNA strands upon integration and excision of the phage DNA.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden.