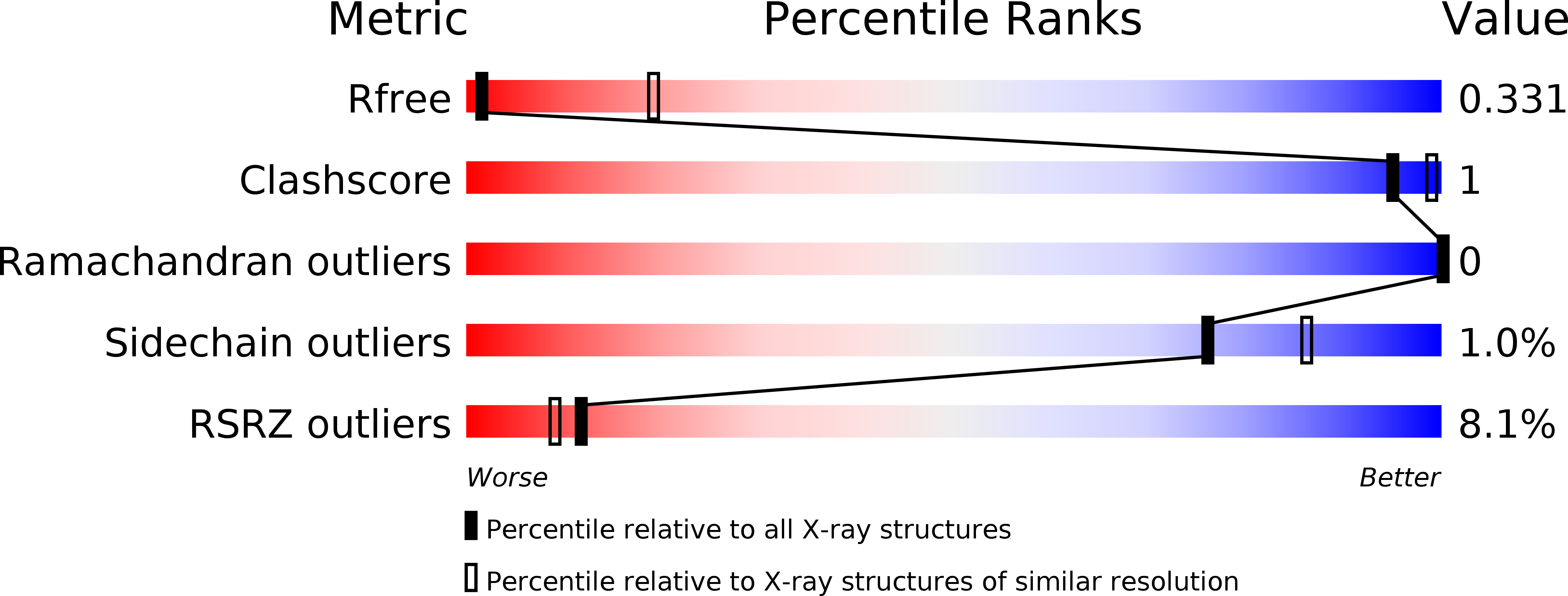
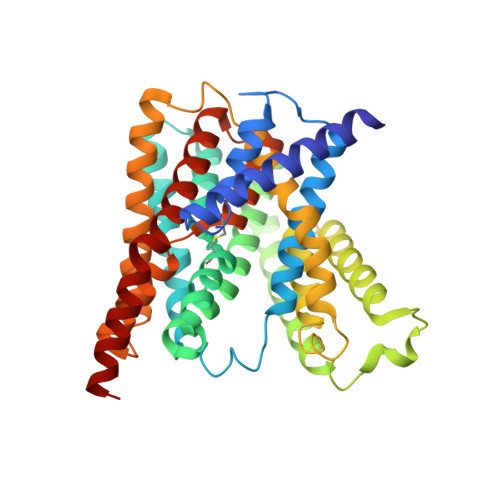
Crystal structures reveal the molecular basis of ion translocation in sodium/proton antiporters.
Coincon, M., Uzdavinys, P., Nji, E., Dotson, D.L., Winkelmann, I., Abdul-Hussein, S., Cameron, A.D., Beckstein, O., Drew, D.(2016) Nat Struct Mol Biol 23: 248-255
- PubMed: 26828964
- DOI: https://doi.org/10.1038/nsmb.3164
- Primary Citation of Related Structures:
5BZ2, 5BZ3 - PubMed Abstract:
To fully understand the transport mechanism of Na(+)/H(+) exchangers, it is necessary to clearly establish the global rearrangements required to facilitate ion translocation. Currently, two different transport models have been proposed. Some reports have suggested that structural isomerization is achieved through large elevator-like rearrangements similar to those seen in the structurally unrelated sodium-coupled glutamate-transporter homolog GltPh. Others have proposed that only small domain movements are required for ion exchange, and a conventional rocking-bundle model has been proposed instead. Here, to resolve these differences, we report atomic-resolution structures of the same Na(+)/H(+) antiporter (NapA from Thermus thermophilus) in both outward- and inward-facing conformations. These data combined with cross-linking, molecular dynamics simulations and isothermal calorimetry suggest that Na(+)/H(+) antiporters provide alternating access to the ion-binding site by using elevator-like structural transitions.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden.