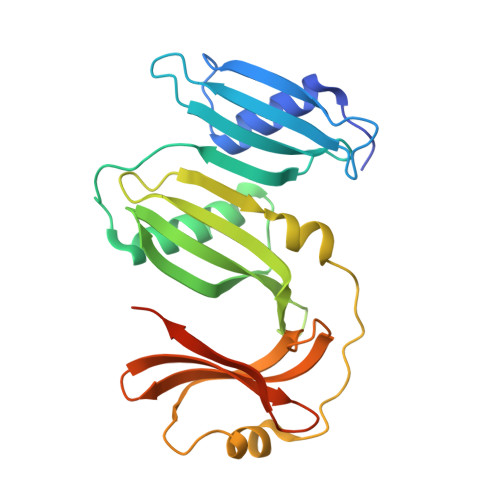
Crystal structure of the PepSY-containing domain of the YpeB protein involved in germination of bacillus spores.
Ustok, F.I., Chirgadze, D.Y., Christie, G.(2015) Proteins 83: 1914-1921
- PubMed: 26219275
- DOI: https://doi.org/10.1002/prot.24868
- Primary Citation of Related Structures:
5BOI - PubMed Abstract:
The crystal structure of the C-terminal domain of the Bacillus megaterium YpeB protein has been solved by X-ray crystallography to 1.80-Å resolution. The full-length protein is essential in stabilising the SleB cortex lytic enzyme in Bacillus spores, and may have a role in regulating SleB activity during spore germination. The YpeB-C crystal structure comprises three tandemly repeated PepSY domains, which are aligned to form an extended laterally compressed molecule. A predominantly positively charged region located in the second PepSY domain may provide a site for protein interactions that are important in stabilising SleB and YpeB within the spore.
Organizational Affiliation:
Department of Chemical Engineering and Biotechnology, Institute of Biotechnology, University of Cambridge, Cambridge, United Kingdom.