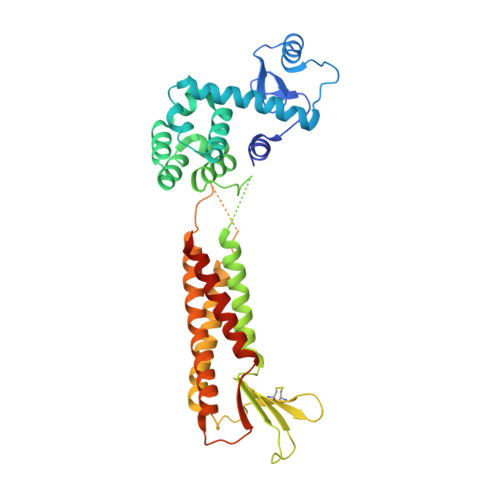
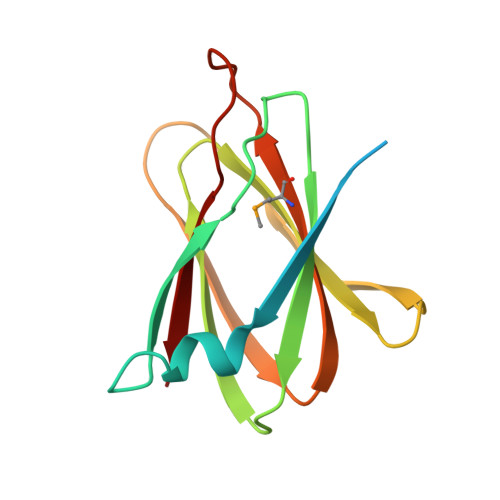
Structural basis for disruption of claudin assembly in tight junctions by an enterotoxin
Shinoda, T., Shinya, N., Ito, K., Ohsawa, N., Terada, T., Hirata, K., Kawano, Y., Yamamoto, M., Kimura-Someya, T., Yokoyama, S., Shirouzu, M.(2016) Sci Rep 6: 33632-33632
- PubMed: 27647526
- DOI: https://doi.org/10.1038/srep33632
- Primary Citation of Related Structures:
5B2G - PubMed Abstract:
The food-poisoning bacterium Clostridium perfringens produces an enterotoxin (~35 kDa) that specifically targets human claudin-4, among the 26 human claudin proteins, and causes diarrhea by fluid accumulation in the intestinal cavity. The C-terminal domain of the Clostridium perfringens enterotoxin (C-CPE, ~15 kDa) binds tightly to claudin-4, and disrupts the intestinal tight junction barriers. In this study, we determined the 3.5-Å resolution crystal structure of the cell-free synthesized human claudin-4•C-CPE complex, which is significantly different from the structure of the off-target complex of an engineered C-CPE with mouse claudin-19. The claudin-4•C-CPE complex structure demonstrated the mechanism underlying claudin assembly disruption. A comparison of the present C-CPE-bound structure of claudin-4 with the enterotoxin-free claudin-15 structure revealed sophisticated C-CPE-induced conformation changes of the extracellular segments, induced on the foundation of the rigid four-transmembrane-helix bundle structure. These conformation changes provide a mechanistic model for the disruption of the lateral assembly of claudin molecules. Furthermore, the present novel structural mechanism for selecting a specific member of the claudin family can be used as the foundation to develop novel medically important technologies to selectively regulate the tight junctions formed by claudin family members in different organs.
Organizational Affiliation:
RIKEN Systems and Structural Biology Center, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama 230-0045, Japan.