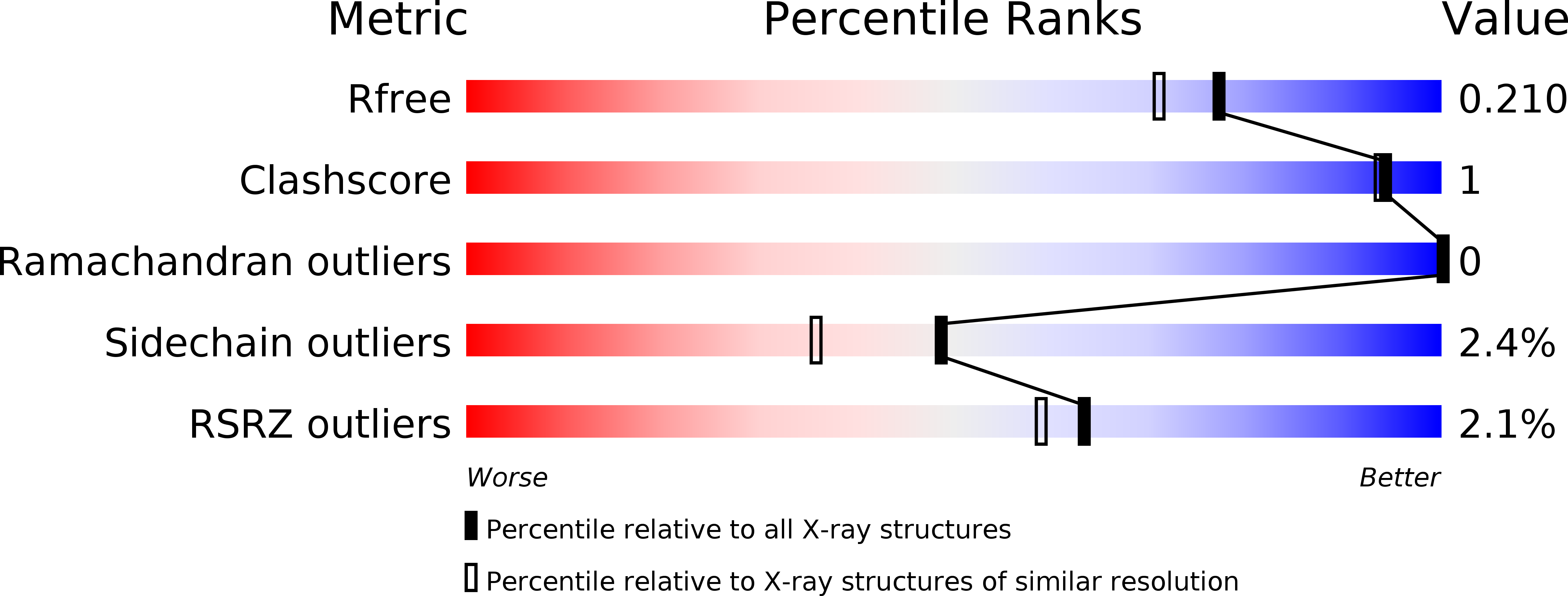
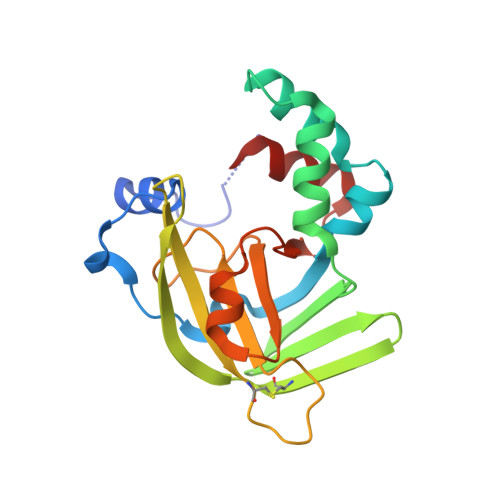
Roles of Escherichia Coli Zint in Cobalt, Mercury and Cadmium Resistance and Structural Insights Into the Metal Binding Mechanism
Colaco, H.G., Santo, P.E., Matias, P., Bandeiras, T.M., Vicente, J.B.(2016) Metallomics 8: 327
- PubMed: 26758285
- DOI: https://doi.org/10.1039/c5mt00291e
- Primary Citation of Related Structures:
5AQ6 - PubMed Abstract:
Escherichia coli ZinT is a metal binding protein involved in zinc homeostasis, with additional putative functions in the resistance against other metals. Herein, a method was designed and implemented to evaluate from a structural and functional viewpoint metal binding to E. coli ZinT in 96-well microtiter plates. The isolated ZinT was mixed with several metal ions and their binding ability was determined by differential scanning fluorimetry. From the positive hits, six metal ions were evaluated in terms of their toxicity towards an E. coli strain depleted of ZinT (ΔzinT) using as control a strain deleted in the galT gene (ΔgalT). The different sensitivities of each strain to the tested metals revealed novel roles of ZinT in the resistance to cobalt, cadmium and mercury. This approach provides a valuable and reliable platform for the analysis of metal binding and its functional implications, extendable to other metal binding proteins. In combination with the developed platform, structural studies were performed with ZinT, with the zinc-loaded crystallographic structure being obtained at 1.79 Å resolution. Besides the canonical zinc-binding site located near the N-terminus, the herein reported dimeric ZinT structure unravelled extra zinc binding sites that support its role in metal loading and/or transport. Altogether, the designed experimental platform allowed revealing new roles for the ZinT protein in microbial resistance to heavy metal toxicity, as well as structural insights into the ZinT metal binding mechanism.
Organizational Affiliation:
Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, University of Lisbon, Portugal.