Crystal Structure and Md Simulation of Mouse Endov Reveal Wedge Motif Plasticity in This Inosine-Specific Endonuclease.
Nawaz, M.S., Vik, E.S., Ronander, M.E., Solvoll, A.M., Blicher, P., Bjoras, M., Alseth, I., Dalhus, B.(2016) Sci Rep 6: 24979
- PubMed: 27108838
- DOI: https://doi.org/10.1038/srep24979
- Primary Citation of Related Structures:
5AOY - PubMed Abstract:
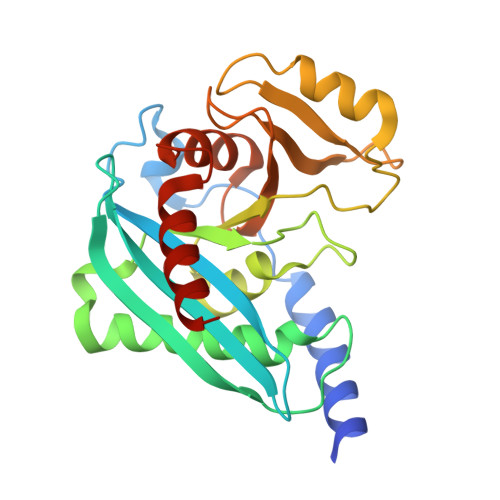
Endonuclease V (EndoV) is an enzyme with specificity for deaminated adenosine (inosine) in nucleic acids. EndoV from Escherichia coli (EcEndoV) acts both on inosines in DNA and RNA, whereas the human homolog cleaves only at inosines in RNA. Inosines in DNA are mutagenic and the role of EndoV in DNA repair is well established. In contrast, the biological function of EndoV in RNA processing is largely unexplored. Here we have characterized a second mammalian EndoV homolog, mouse EndoV (mEndoV), and show that mEndoV shares the same RNA selectivity as human EndoV (hEndoV). Mouse EndoV cleaves the same inosine-containing substrates as hEndoV, but with reduced efficiencies. The crystal structure of mEndoV reveals a conformation different from the hEndoV and prokaryotic EndoV structures, particularly for the conserved tyrosine in the wedge motif, suggesting that this strand separating element has some flexibility. Molecular dynamics simulations of mouse and human EndoV reveal alternative conformations for the invariant tyrosine. The configuration of the active site, on the other hand, is very similar between the prokaryotic and mammalian versions of EndoV.
Organizational Affiliation:
Department of Microbiology, Oslo University Hospital HF, Rikshospitalet and University of Oslo, NO-0424 Oslo, Norway.