Substituted 2-(2-Aminopyrimidin-4-Yl)Pyridine-4-Carboxylates as Potent Inhibitors of Jumonjic Domain-Containing Histone Demethylases.
Roatsch, M., Robaa, D., Pippel, M., Nettleship, J.E., Reddivari, Y., Bird, L.E., Hoffmann, I., Franz, H., Owens, R.J., Schole, R., Flaig, R., Sippl, W., Jung, M.(2016) Future Med Chem 8: 1553
- PubMed: 26971619
- DOI: https://doi.org/10.4155/fmc.15.188
- Primary Citation of Related Structures:
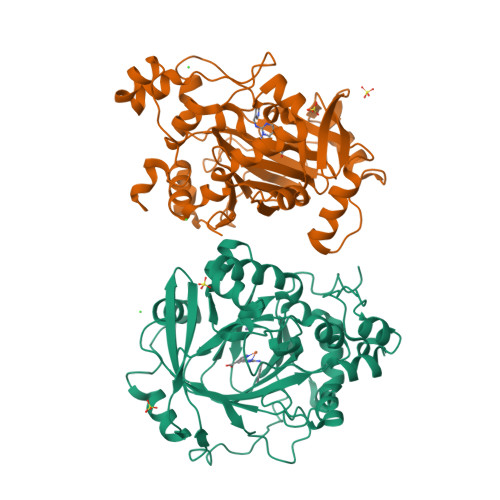
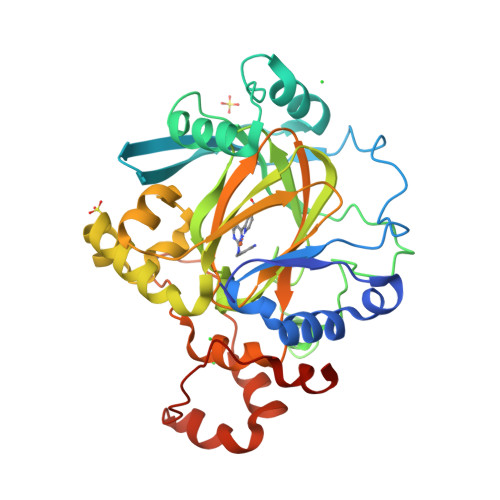
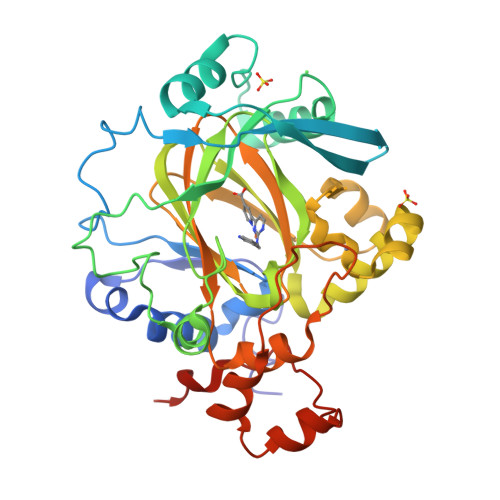
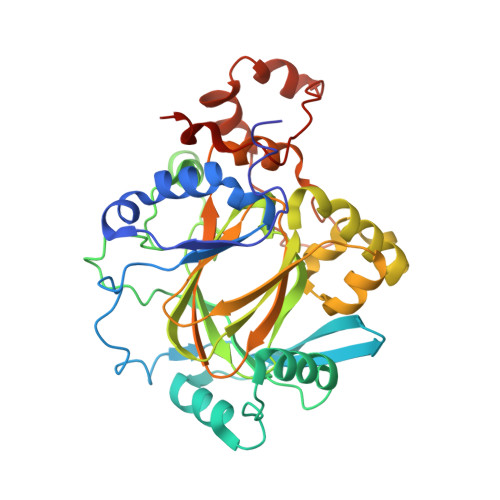
5ANQ - PubMed Abstract:
Aberrant expression of iron(II)- and 2-oxoglutarate-dependent JumonjiC histone demethylases has been linked to cancer. Potent demethylase inhibitors are drug candidates and biochemical tools to elucidate the functional impact of demethylase inhibition. Virtual screening identified a novel lead scaffold against JMJD2A with low-micromolar potency in vitro. Analogs were acquired from commercial sources respectively synthesized in feedback with biological testing. Optimized compounds were transformed into cell-permeable prodrugs. A cocrystal x-ray structure revealed the mode of binding of these compounds as competitive to 2-oxoglutarate and confirmed kinetic experiments. Selectivity studies revealed a preference for JMJD2A and JARID1A over JMJD3. Virtual screening and rational structural optimization led to a novel scaffold for highly potent and selective JMJD2A inhibitors.
Organizational Affiliation:
Albert-Ludwigs-University Freiburg, Institute of Pharmaceutical Sciences, Albertstraße 25, 79104 Freiburg i.Br., Germany.