Structural Basis for the Ring Catalyzed Synthesis of K63 Linked Ubiquitin Chains
Branigan, E., Plechanovova, A., Jaffray, E., Naismith, J.H., Hay, R.T.(2015) Nat Struct Mol Biol 22: 597
- PubMed: 26148049
- DOI: https://doi.org/10.1038/nsmb.3052
- Primary Citation of Related Structures:
5AIT, 5AIU - PubMed Abstract:
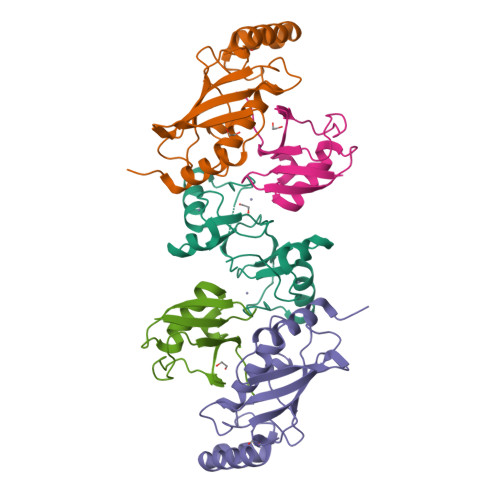
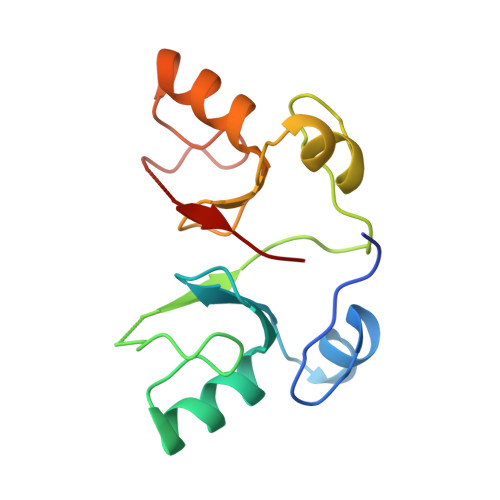
RING E3 ligase-catalyzed formation of K63-linked ubiquitin chains by the Ube2V2-Ubc13 E2 complex is required in many important biological processes. Here we report the structure of the RING-domain dimer of rat RNF4 in complex with a human Ubc13∼Ub conjugate and Ube2V2. The structure has captured Ube2V2 bound to the acceptor (priming) ubiquitin with K63 in a position favorable for attack on the linkage between Ubc13 and the donor (second) ubiquitin held in the active 'folded back' conformation by the RING domain of RNF4. We verified the interfaces identified in the structure by in vitro ubiquitination assays of site-directed mutants. To our knowledge, this represents the first view of synthesis of K63-linked ubiquitin chains in which both substrate ubiquitin and ubiquitin-loaded E2 are juxtaposed to allow E3 ligase-mediated catalysis.
Organizational Affiliation:
Centre for Gene Regulation and Expression, University of Dundee, Dundee, UK.