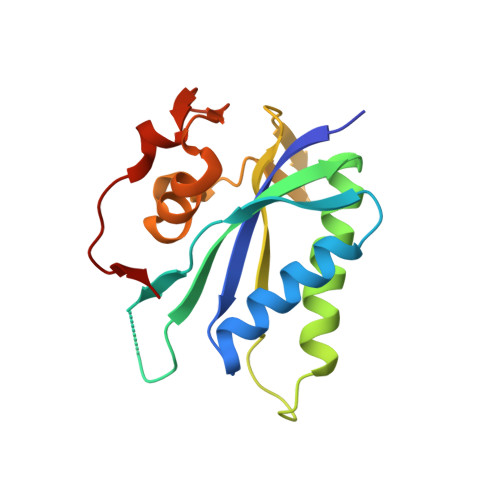
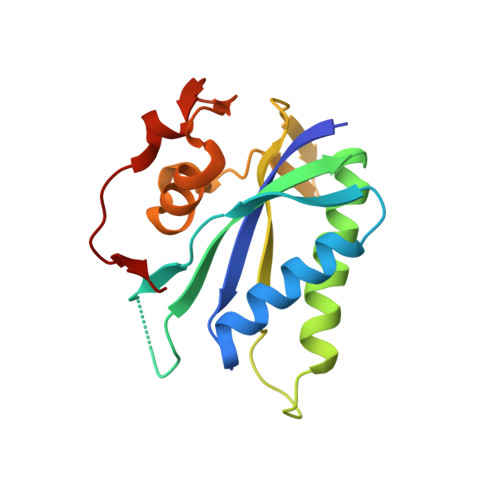
Structure-Based Design and Development of Functionalized Mercaptoguanine Derivatives as Inhibitors of the Folate Biosynthesis Pathway Enzyme 6-Hydroxymethyl-7,8-Dihydropterin Pyrophosphokinase from Staphylococcus Aureus.
Dennis, M.L., Chhabra, S., Wang, Z., Debono, A., Dolezal, O., Newman, J., Pitcher, N.P., Rahmani, R., Cleary, B., Barlow, N., Hattarki, M., Graham, B., Peat, T.S., Baell, J.B., Swarbrick, J.D.(2014) J Med Chem 57: 9612
- PubMed: 25357262
- DOI: https://doi.org/10.1021/jm501417f
- Primary Citation of Related Structures:
4CRJ, 4CWB, 4CYU - PubMed Abstract:
6-Hydroxymethyl-7,8-dihydropterin pyrophosphokinase (HPPK), an enzyme from the folate biosynthesis pathway, catalyzes the pyrophosphoryl transfer from ATP to 6-hydroxymethyl-7,8-dihydropterin and is a yet-to-be-drugged antimicrobial target. Building on our previous discovery that 8-mercaptoguanine (8MG) is an inhibitor of Staphylococcus aureus HPPK (SaHPPK), we have identified and characterized the binding of an S8-functionalized derivative (3). X-ray structures of both the SaHPPK/3/cofactor analogue ternary and the SaHPPK/cofactor analogue binary complexes have provided insight into cofactor recognition and key residues that move over 30 Å upon binding of 3, whereas NMR measurements reveal a partially plastic ternary complex active site. Synthesis and binding analysis of a set of analogues of 3 have identified an advanced new lead compound (11) displaying >20-fold higher affinity for SaHPPK than 8MG. A number of these exhibited low micromolar affinity for dihydropteroate synthase (DHPS), the adjacent, downstream enzyme to HPPK, and may thus represent promising new leads to bienzyme inhibitors.
- Monash Institute of Pharmaceutical Sciences, Monash University , Parkville, Victoria 3052, Australia.
Organizational Affiliation: