DNA recognition for virus assembly through multiple sequence-independent interactions with a helix-turn-helix motif.
Greive, S.J., Fung, H.K., Chechik, M., Jenkins, H.T., Weitzel, S.E., Aguiar, P.M., Brentnall, A.S., Glousieau, M., Gladyshev, G.V., Potts, J.R., Antson, A.A.(2016) Nucleic Acids Res 44: 776-789
- PubMed: 26673721
- DOI: https://doi.org/10.1093/nar/gkv1467
- Primary Citation of Related Structures:
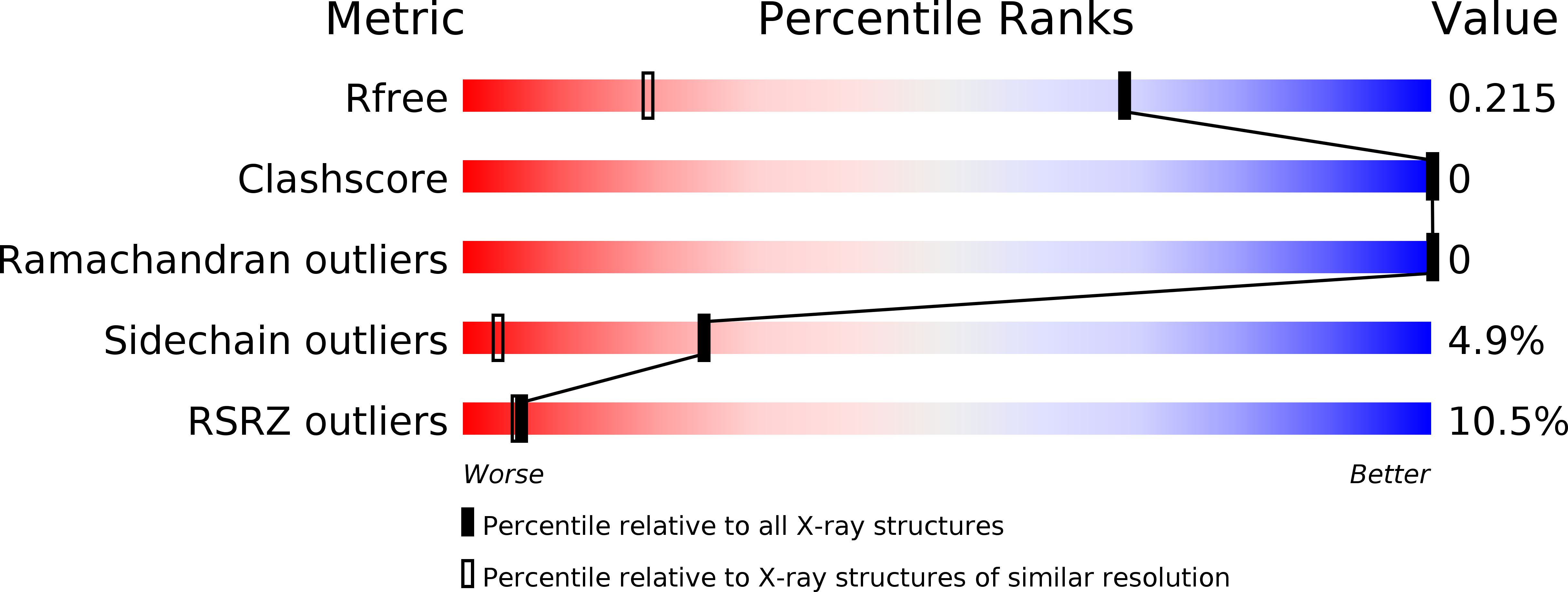
4ZC3 - PubMed Abstract:
The helix-turn-helix (HTH) motif features frequently in protein DNA-binding assemblies. Viral pac site-targeting small terminase proteins possess an unusual architecture in which the HTH motifs are displayed in a ring, distinct from the classical HTH dimer. Here we investigate how such a circular array of HTH motifs enables specific recognition of the viral genome for initiation of DNA packaging during virus assembly. We found, by surface plasmon resonance and analytical ultracentrifugation, that individual HTH motifs of the Bacillus phage SF6 small terminase bind the packaging regions of SF6 and related SPP1 genome weakly, with little local sequence specificity. Nuclear magnetic resonance chemical shift perturbation studies with an arbitrary single-site substrate suggest that the HTH motif contacts DNA similarly to how certain HTH proteins contact DNA non-specifically. Our observations support a model where specificity is generated through conformational selection of an intrinsically bent DNA segment by a ring of HTHs which bind weakly but cooperatively. Such a system would enable viral gene regulation and control of the viral life cycle, with a minimal genome, conferring a major evolutionary advantage for SPP1-like viruses.
Organizational Affiliation:
York Structural Biology Laboratory, Department of Chemistry, University of York, York YO10 5DD, UK.