MupS, a 3-oxoacyl (ACP) reductase involved in Mupirocin biosynthesis
Till, M., Race, P.R.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| MupS | 257 | Pseudomonas fluorescens | Mutation(s): 0 Gene Names: mupS | 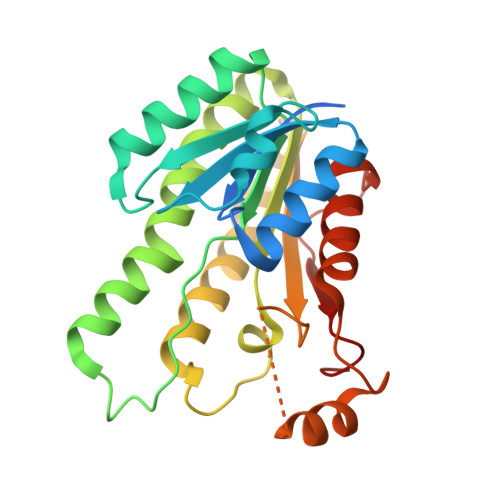 | |
UniProt | |||||
Find proteins for Q8RL53 (Pseudomonas fluorescens) Explore Q8RL53 Go to UniProtKB: Q8RL53 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8RL53 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 94.08 | α = 90 |
| b = 94.08 | β = 90 |
| c = 194.341 | γ = 120 |
| Software Name | Purpose |
|---|---|
| SCALA | data scaling |
| PHASER | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| MOSFLM | data reduction |
| Coot | model building |