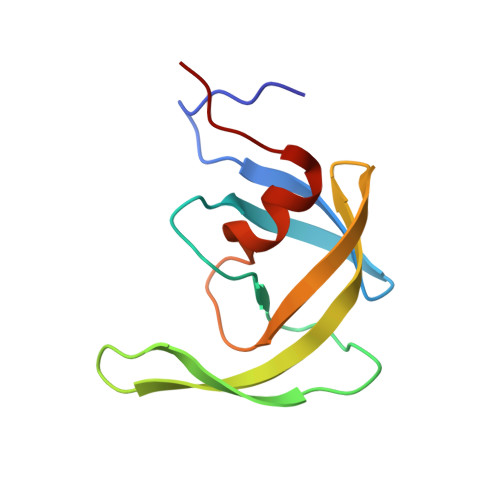
The L33F darunavir resistance mutation acts as a molecular anchor reducing the flexibility of the HIV-1 protease 30s and 80s loops.
Kuiper, B.D., Keusch, B.J., Dewdney, T.G., Chordia, P., Ross, K., Brunzelle, J.S., Kovari, I.A., MacArthur, R., Salimnia, H., Kovari, L.C.(2015) Biochem Biophys Rep 2: 160-165
- PubMed: 29124158
- DOI: https://doi.org/10.1016/j.bbrep.2015.06.003
- Primary Citation of Related Structures:
4YOA, 4YOB - PubMed Abstract:
HIV-1 protease (PR) is a 99 amino acid protein responsible for proteolytic processing of the viral polyprotein - an essential step in the HIV-1 life cycle. Drug resistance mutations in PR that are selected during antiretroviral therapy lead to reduced efficacy of protease inhibitors (PI) including darunavir (DRV). To identify the structural mechanisms associated with the DRV resistance mutation L33F, we performed X-ray crystallographic studies with a multi-drug resistant HIV-1 protease isolate that contains the L33F mutation (MDR769 L33F). In contrast to other PR L33F DRV complexes, the structure of MDR769 L33F complexed with DRV reported here displays the protease flaps in an open conformation. The L33F mutation increases noncovalent interactions in the hydrophobic pocket of the PR compared to the wild-type (WT) structure. As a result, L33F appears to act as a molecular anchor, reducing the flexibility of the 30s loop (residues 29-35) and the 80s loop (residues 79-84). Molecular anchoring of the 30s and 80s loops leaves an open S1/S1' subsite and distorts the conserved hydrogen-bonding network of DRV. These findings are consistent with previous reports despite structural differences with regards to flap conformation.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Wayne State University School of Medicine, Detroit, MI, USA.