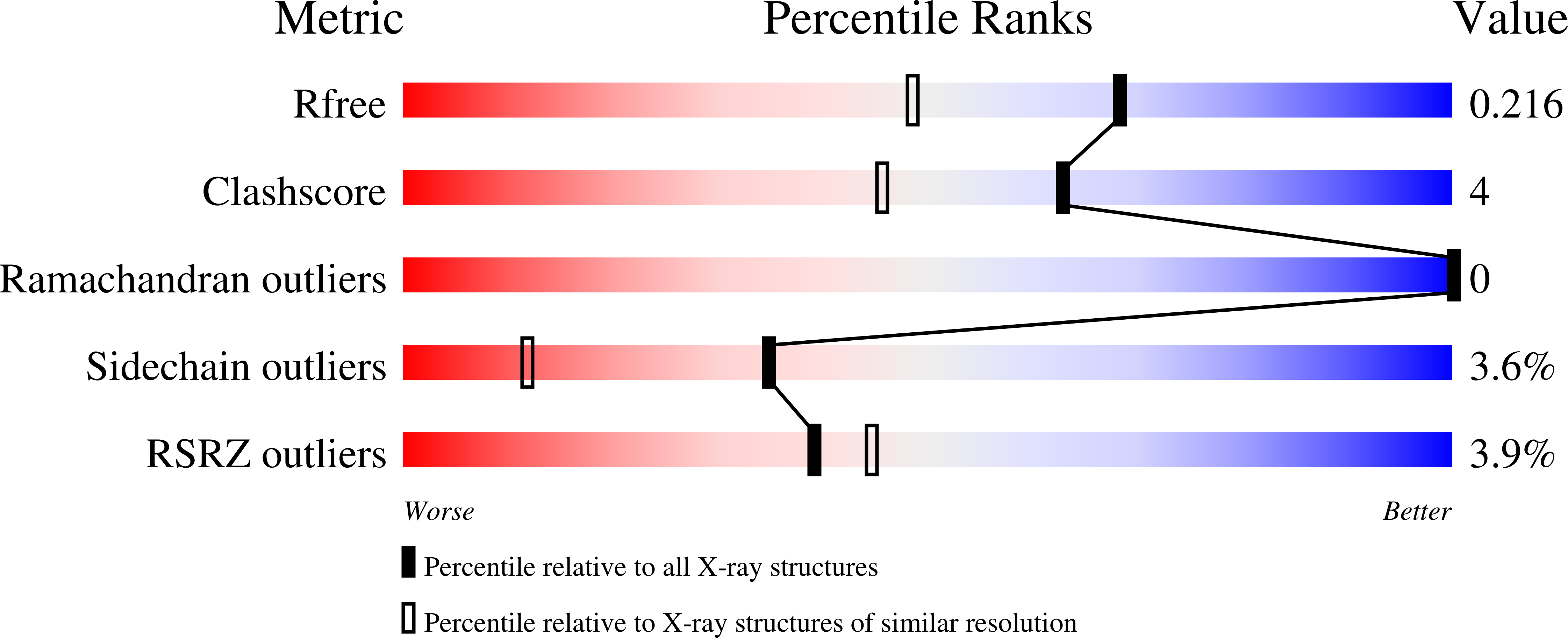
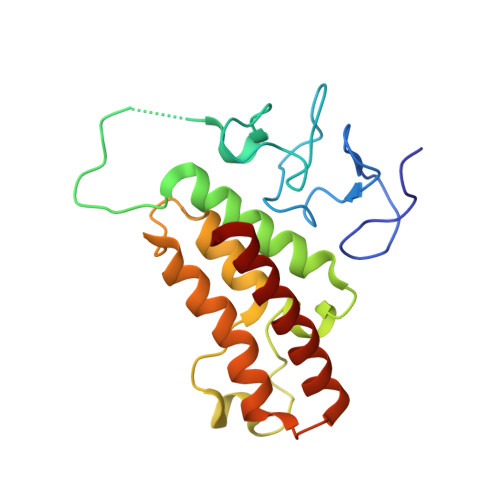
Structure-Guided Design of IACS-9571, a Selective High-Affinity Dual TRIM24-BRPF1 Bromodomain Inhibitor.
Palmer, W.S., Poncet-Montange, G., Liu, G., Petrocchi, A., Reyna, N., Subramanian, G., Theroff, J., Yau, A., Kost-Alimova, M., Bardenhagen, J.P., Leo, E., Shepard, H.E., Tieu, T.N., Shi, X., Zhan, Y., Zhao, S., Barton, M.C., Draetta, G., Toniatti, C., Jones, P., Geck Do, M., Andersen, J.N.(2016) J Med Chem 59: 1440-1454
- PubMed: 26061247
- DOI: https://doi.org/10.1021/acs.jmedchem.5b00405
- Primary Citation of Related Structures:
4YAB, 4YAD, 4YAT, 4YAX, 4YBM, 4YBS, 4YBT, 4YC9 - PubMed Abstract:
The bromodomain containing proteins TRIM24 (tripartite motif containing protein 24) and BRPF1 (bromodomain and PHD finger containing protein 1) are involved in the epigenetic regulation of gene expression and have been implicated in human cancer. Overexpression of TRIM24 correlates with poor patient prognosis, and BRPF1 is a scaffolding protein required for the assembly of histone acetyltransferase complexes, where the gene of MOZ (monocytic leukemia zinc finger protein) was first identified as a recurrent fusion partner in leukemia patients (8p11 chromosomal rearrangements). Here, we present the structure guided development of a series of N,N-dimethylbenzimidazolone bromodomain inhibitors through the iterative use of X-ray cocrystal structures. A unique binding mode enabled the design of a potent and selective inhibitor 8i (IACS-9571) with low nanomolar affinities for TRIM24 and BRPF1 (ITC Kd = 31 nM and ITC Kd = 14 nM, respectively). With its excellent cellular potency (EC50 = 50 nM) and favorable pharmacokinetic properties (F = 29%), 8i is a high-quality chemical probe for the evaluation of TRIM24 and/or BRPF1 bromodomain function in vitro and in vivo.
Organizational Affiliation:
Department of Epigenetics and Molecular Carcinogenesis, The University of Texas MD Anderson Cancer Center , 1515 Holcombe Boulevard , Houston, Texas 77030, United States.