Structure of the catalytic phosphatase domain of MTMR8: implications for dimerization, membrane association and reversible oxidation.
Yoo, K.Y., Son, J.Y., Lee, J.U., Shin, W., Im, D.W., Kim, S.J., Ryu, S.E., Heo, Y.S.(2015) Acta Crystallogr D Biol Crystallogr 71: 1528-1539
- PubMed: 26143924
- DOI: https://doi.org/10.1107/S139900471500927X
- Primary Citation of Related Structures:
4Y7I - PubMed Abstract:
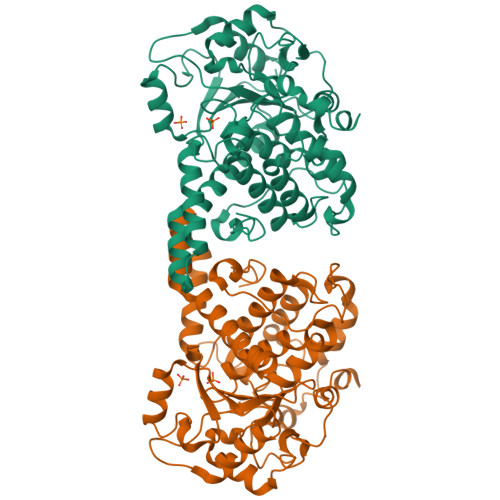
Myotubularin-related proteins are a large family of phosphoinositide phosphatases; their activity, stability and subcellular localization are regulated by dimeric interactions with other members of the family. Here, the crystal structure of the phosphatase domain of MTMR8 is reported. Conformational deviation of the two loops that mediate interaction with the PH-GRAM domain suggests that the PH-GRAM domain interacts differently with the phosphatase domain of each MTMR member. The protein exists as a dimer with twofold symmetry, providing insight into a novel mode of dimerization mediated by the phosphatase domain. Structural comparison and mutation studies suggest that Lys255 of MTMR8 interacts with the substrate diacylglycerol moiety, similar to Lys333 of MTMR2, although the positions of these residues are different. The catalytic activity of the MTMR8 phosphatase domain is inhibited by oxidation and is reversibly reactivated by reduction, suggesting the presence of an oxidation-protective intermediate other than a disulfide bond owing to the absence of a cysteine within a disulfide-bond distance from Cys338.
Organizational Affiliation:
Department of Chemistry, Konkuk University, 1 Hwayang-dong, Gwangjin-gu, Seoul 143-701, Republic of Korea.