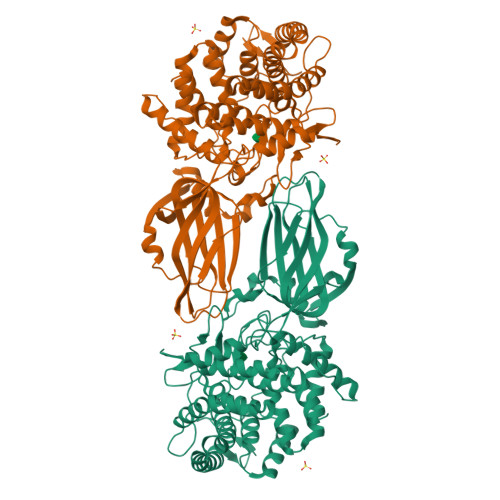
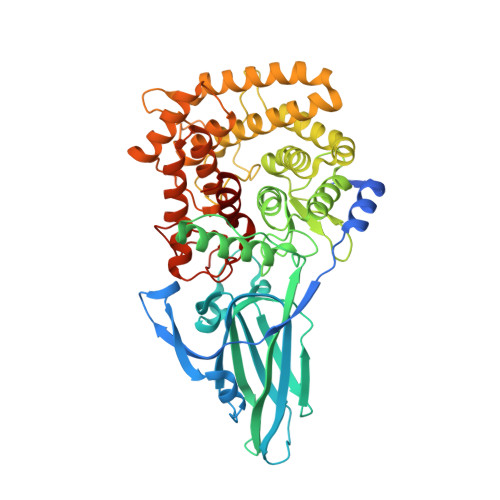
Crystal structure of a novel two domain GH78 family alpha-rhamnosidase from Klebsiella oxytoca with rhamnose bound.
O'Neill, E.C., Stevenson, C.E., Paterson, M.J., Rejzek, M., Chauvin, A.L., Lawson, D.M., Field, R.A.(2015) Proteins 83: 1742-1749
- PubMed: 25846411
- DOI: https://doi.org/10.1002/prot.24807
- Primary Citation of Related Structures:
4XHC - PubMed Abstract:
The crystal structure of the GH78 family α-rhamnosidase from Klebsiella oxytoca (KoRha) has been determined at 2.7 Å resolution with rhamnose bound in the active site of the catalytic domain. Curiously, the putative catalytic acid, Asp 222, is preceded by an unusual non-proline cis-peptide bond which helps to project the carboxyl group into the active centre. This KoRha homodimeric structure is significantly smaller than those of the other previously determined GH78 structures. Nevertheless, the enzyme displays α-rhamnosidase activity when assayed in vitro, suggesting that the additional structural domains found in the related enzymes are dispensible for function.
Organizational Affiliation:
Department of Biological Chemistry, John Innes Centre, Norwich Research Park, Norwich, Nr4 7UH, United Kingdom.