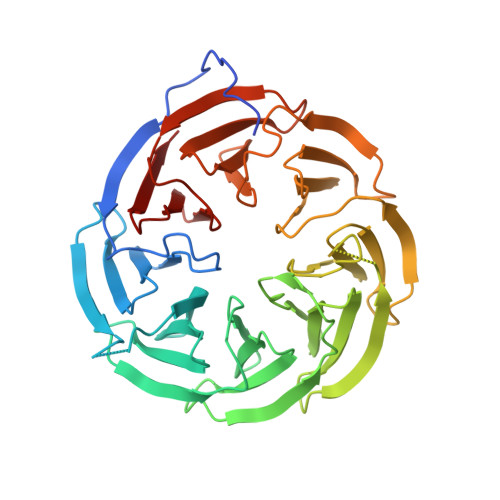
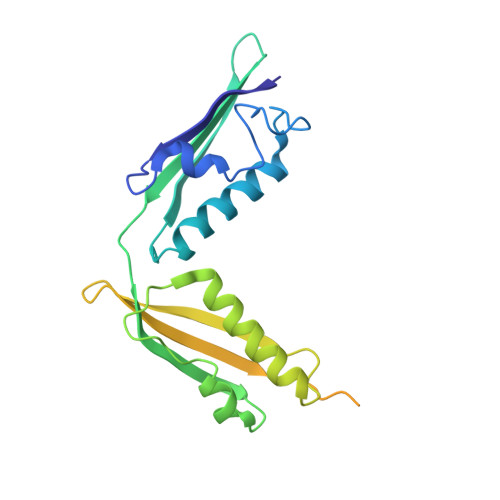
Structural basis for the interaction of BamB with the POTRA3-4 domains of BamA.
Chen, Z., Zhan, L.H., Hou, H.F., Gao, Z.Q., Xu, J.H., Dong, C., Dong, Y.H.(2016) Acta Crystallogr D Struct Biol 72: 236-244
- PubMed: 26894671
- DOI: https://doi.org/10.1107/S2059798315024729
- Primary Citation of Related Structures:
4XGA - PubMed Abstract:
In Escherichia coli, the Omp85 protein BamA and four lipoproteins (BamBCDE) constitute the BAM complex, which is essential for the assembly and insertion of outer membrane proteins into the outer membrane. Here, the crystal structure of BamB in complex with the POTRA3-4 domains of BamA is reported at 2.1 Å resolution. Based on this structure, the POTRA3 domain is associated with BamB via hydrogen-bonding and hydrophobic interactions. Structural and biochemical analysis revealed that the conserved residues Arg77, Glu127, Glu150, Ser167, Leu192, Leu194 and Arg195 of BamB play an essential role in interaction with the POTRA3 domain.
Organizational Affiliation:
Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, People's Republic of China.