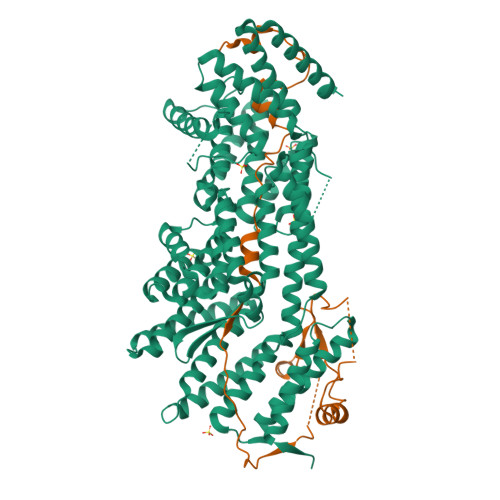
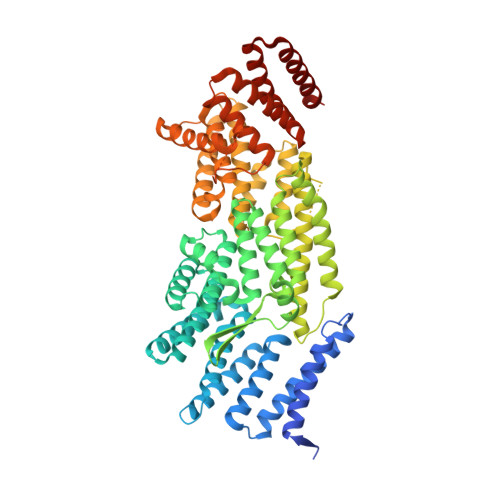
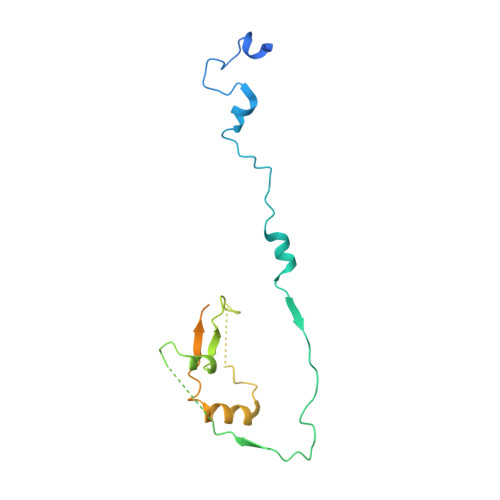
Structural evidence for Scc4-dependent localization of cohesin loading.
Hinshaw, S.M., Makrantoni, V., Kerr, A., Marston, A.L., Harrison, S.C.(2015) Elife 4: e06057-e06057
- PubMed: 26038942
- DOI: https://doi.org/10.7554/eLife.06057
- Primary Citation of Related Structures:
4XDN - PubMed Abstract:
The cohesin ring holds newly replicated sister chromatids together until their separation at anaphase. Initiation of sister chromatid cohesion depends on a separate complex, Scc2(NIPBL)/Scc4(Mau2) (Scc2/4), which loads cohesin onto DNA and determines its localization across the genome. Proper cohesin loading is essential for cell division, and partial defects cause chromosome missegregation and aberrant transcriptional regulation, leading to severe developmental defects in multicellular organisms. We present here a crystal structure showing the interaction between Scc2 and Scc4. Scc4 is a TPR array that envelops an extended Scc2 peptide. Using budding yeast, we demonstrate that a conserved patch on the surface of Scc4 is required to recruit Scc2/4 to centromeres and to build pericentromeric cohesion. These findings reveal the role of Scc4 in determining the localization of cohesin loading and establish a molecular basis for Scc2/4 recruitment to centromeres.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, United States.