The Discovery and Characterization of the alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptor Potentiator N-{(3S,4S)-4-[4-(5-Cyano-2-thienyl)phenoxy]tetrahydrofuran-3-yl}propane-2-sulfonamide (PF-04958242).
Shaffer, C.L., Patel, N.C., Schwarz, J., Scialis, R.J., Wei, Y., Hou, X.J., Xie, L., Karki, K., Bryce, D.K., Osgood, S.M., Hoffmann, W.E., Lazzaro, J.T., Chang, C., McGinnis, D.F., Lotarski, S.M., Liu, J., Obach, R.S., Weber, M.L., Chen, L., Zasadny, K.R., Seymour, P.A., Schmidt, C.J., Hajos, M., Hurst, R.S., Pandit, J., O'Donnell, C.J.(2015) J Med Chem 58: 4291-4308
- PubMed: 25905800
- DOI: https://doi.org/10.1021/acs.jmedchem.5b00300
- Primary Citation of Related Structures:
4X48 - PubMed Abstract:
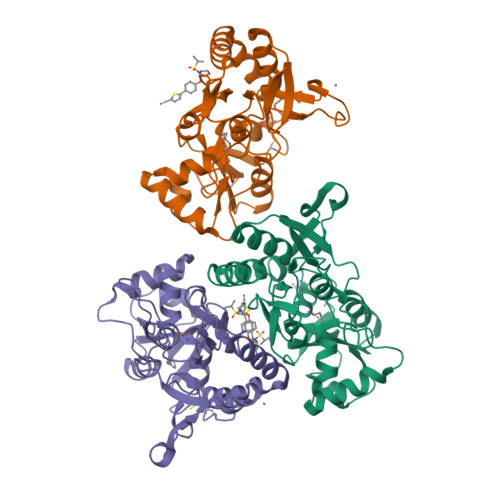
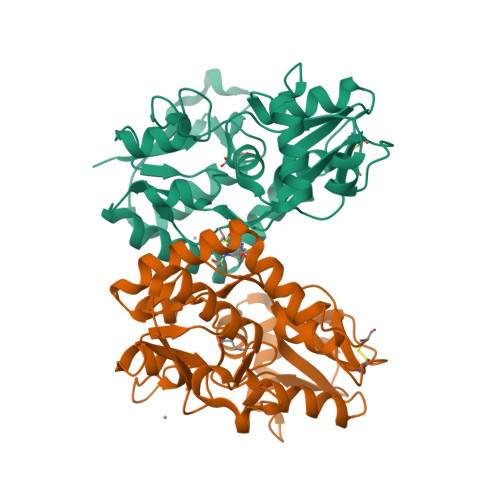
A unique tetrahydrofuran ether class of highly potent α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor potentiators has been identified using rational and structure-based drug design. An acyclic lead compound, containing an ether-linked isopropylsulfonamide and biphenyl group, was pharmacologically augmented by converting it to a conformationally constrained tetrahydrofuran to improve key interactions with the human GluA2 ligand-binding domain. Subsequent replacement of the distal phenyl motif with 2-cyanothiophene to enhance its potency, selectivity, and metabolic stability afforded N-{(3S,4S)-4-[4-(5-cyano-2-thienyl)phenoxy]tetrahydrofuran-3-yl}propane-2-sulfonamide (PF-04958242, 3), whose preclinical characterization suggests an adequate therapeutic index, aided by low projected human oral pharmacokinetic variability, for clinical studies exploring its ability to attenuate cognitive deficits in patients with schizophrenia.