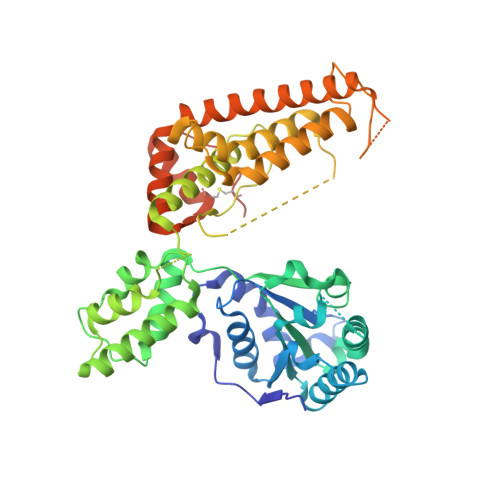
The structure of Aquifex aeolicus FtsH in the ADP-bound state reveals a C2-symmetric hexamer.
Vostrukhina, M., Popov, A., Brunstein, E., Lanz, M.A., Baumgartner, R., Bieniossek, C., Schacherl, M., Baumann, U.(2015) Acta Crystallogr D Biol Crystallogr 71: 1307-1318
- PubMed: 26057670
- DOI: https://doi.org/10.1107/S1399004715005945
- Primary Citation of Related Structures:
4WW0, 4Z8X - PubMed Abstract:
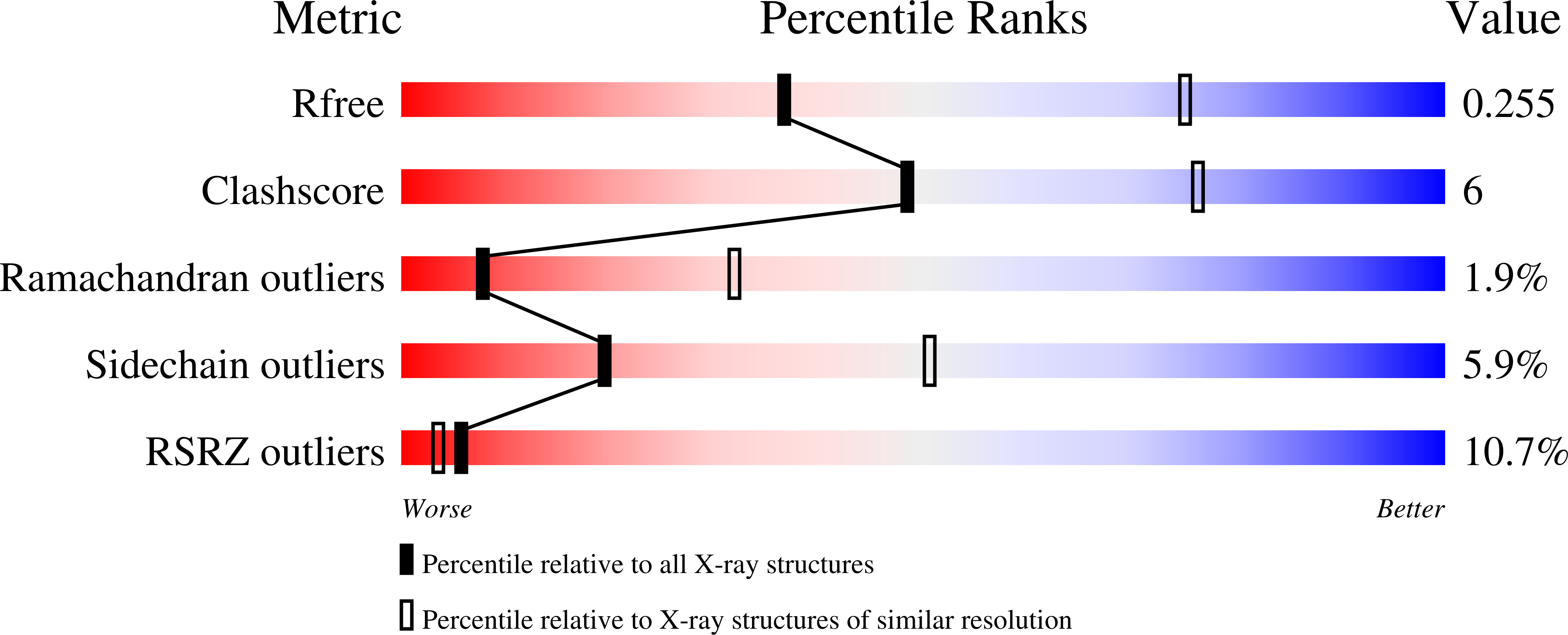
The crystal structure of a truncated, soluble quadruple mutant of FtsH from Aquifex aeolicus comprising the AAA and protease domains has been determined at 2.96 Å resolution in space group I222. The protein crystallizes as a hexamer, with the protease domain forming layers in the ab plane. Contacts between these layers are mediated by the AAA domains. These are highly disordered in one crystal form, but are clearly visible in a related form with a shorter c axis. Here, adenosine diphosphate (ADP) is bound to each subunit and the AAA ring exhibits twofold symmetry. The arrangement is different from the ADP-bound state of an analogously truncated, soluble FtsH construct from Thermotoga maritima. The pore is completely closed and the phenylalanine residues in the pore line a contiguous path. The protease hexamer is very similar to those described for other FtsH structures. To resolve certain open issues regarding a conserved glycine in the linker between the AAA and protease domains, as well as the active-site switch β-strand, mutations have been introduced in the full-length membrane-bound protein. Activity analysis of these point mutants reveals the crucial importance of these residues for proteolytic activity and is in accord with previous interpretation of the active-site switch and the importance of the linker glycine residue.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, Bern, CH-3012, Switzerland.