Structural plasticity in Mycobacterium tuberculosis uracil-DNA glycosylase (MtUng) and its functional implications.
Arif, S.M., Geethanandan, K., Mishra, P., Surolia, A., Varshney, U., Vijayan, M.(2015) Acta Crystallogr D Biol Crystallogr 71: 1514-1527
- PubMed: 26143923
- DOI: https://doi.org/10.1107/S1399004715009311
- Primary Citation of Related Structures:
4WPK, 4WPL, 4WRU, 4WRV, 4WRW, 4WRX, 4WRY, 4WRZ, 4WS0, 4WS1, 4WS2, 4WS3, 4WS4, 4WS5, 4WS6, 4WS7, 4WS8 - PubMed Abstract:
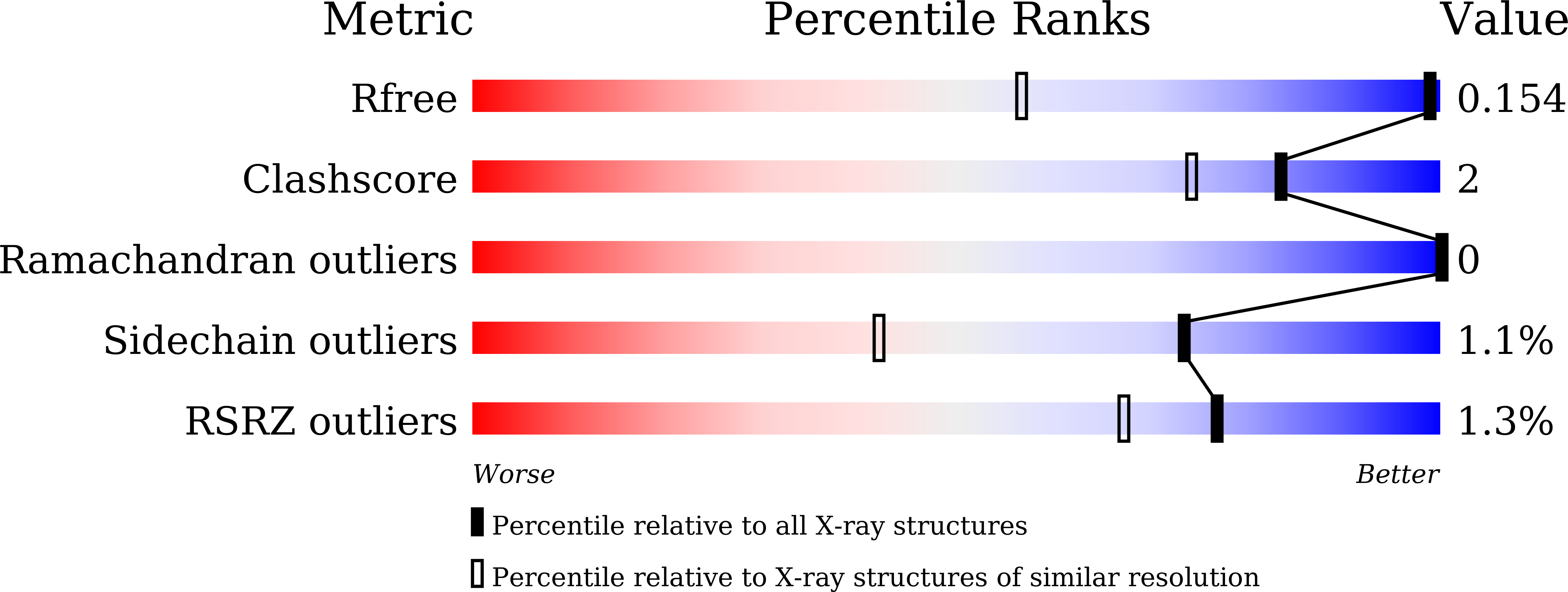
17 independent crystal structures of family I uracil-DNA glycosylase from Mycobacterium tuberculosis (MtUng) and its complexes with uracil and its derivatives, distributed among five distinct crystal forms, have been determined. Thermodynamic parameters of binding in the complexes have been measured using isothermal titration calorimetry. The two-domain protein exhibits open and closed conformations, suggesting that the closure of the domain on DNA binding involves conformational selection. Segmental mobility in the enzyme molecule is confined to a 32-residue stretch which plays a major role in DNA binding. Uracil and its derivatives can bind to the protein in two possible orientations. Only one of them is possible when there is a bulky substituent at the 5' position. The crystal structures of the complexes provide a reasonable rationale for the observed thermodynamic parameters. In addition to providing fresh insights into the structure, plasticity and interactions of the protein molecule, the results of the present investigation provide a platform for structure-based inhibitor design.
Organizational Affiliation:
Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012, India.