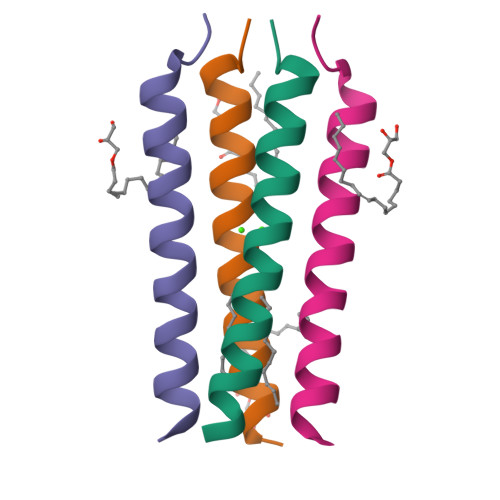
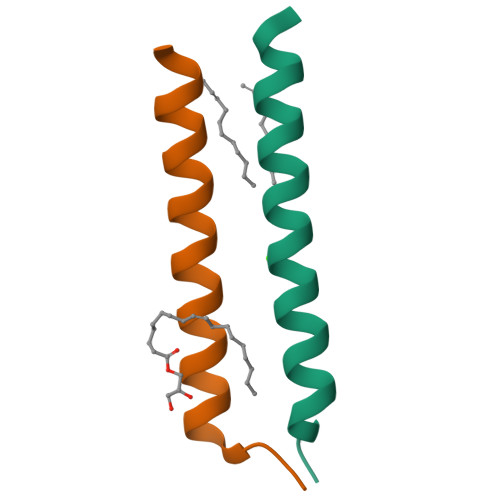
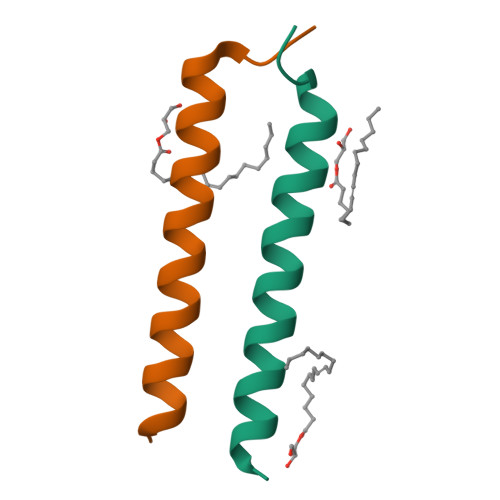
Transmembrane Complexes of DAP12 Crystallized in Lipid Membranes Provide Insights into Control of Oligomerization in Immunoreceptor Assembly.
Knoblich, K., Park, S., Lutfi, M., van 't Hag, L., Conn, C.E., Seabrook, S.A., Newman, J., Czabotar, P.E., Im, W., Call, M.E., Call, M.J.(2015) Cell Rep 11: 1184-1192
- PubMed: 25981043
- DOI: https://doi.org/10.1016/j.celrep.2015.04.045
- Primary Citation of Related Structures:
4WO1, 4WOL - PubMed Abstract:
The membrane-spanning α helices of single-pass receptors play crucial roles in stabilizing oligomeric structures and transducing biochemical signals across the membrane. Probing intermolecular transmembrane interactions in single-pass receptors presents unique challenges, reflected in a gross underrepresentation of their membrane-embedded domains in structural databases. Here, we present two high-resolution structures of transmembrane assemblies from a eukaryotic single-pass protein crystallized in a lipidic membrane environment. Trimeric and tetrameric structures of the immunoreceptor signaling module DAP12, determined to 1.77-Å and 2.14-Å resolution, respectively, are organized by the same polar surfaces that govern intramembrane assembly with client receptors. We demonstrate that, in addition to the well-studied dimeric form, these trimeric and tetrameric structures are made in cells, and their formation is competitive with receptor association in the ER. The polar transmembrane sequences therefore act as primary determinants of oligomerization specificity through interplay between charge shielding and sequestration of polar surfaces within helix interfaces.
Organizational Affiliation:
Structural Biology Division, The Walter and Eliza Hall Institute of Medical Research, Parkville, VIC 3052, Australia; Department of Medical Biology, The University of Melbourne, Parkville, VIC 3052, Australia.