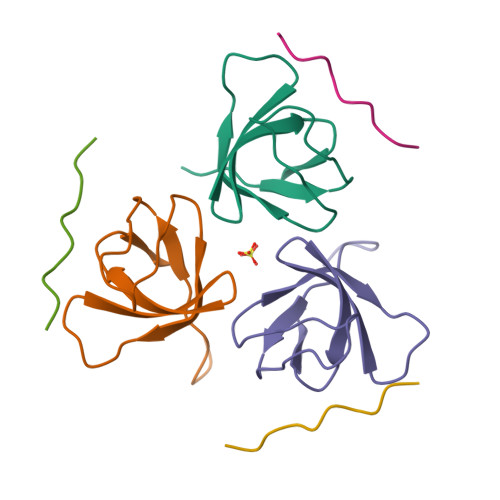
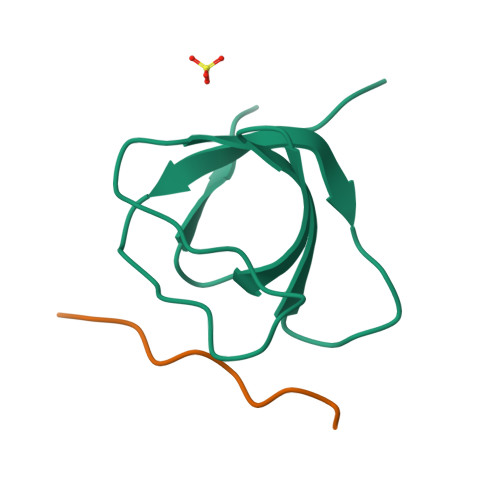
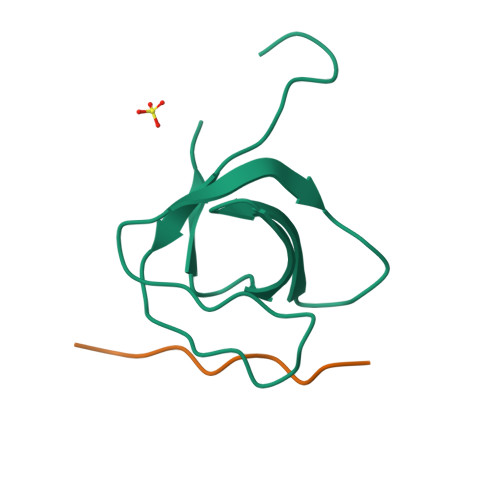
Differential Recognition Preferences of the Three Src Homology 3 (SH3) Domains from the Adaptor CD2-associated Protein (CD2AP) and Direct Association with Ras and Rab Interactor 3 (RIN3).
Rouka, E., Simister, P.C., Janning, M., Kumbrink, J., Konstantinou, T., Muniz, J.R., Joshi, D., O'Reilly, N., Volkmer, R., Ritter, B., Knapp, S., von Delft, F., Kirsch, K.H., Feller, S.M.(2015) J Biological Chem 290: 25275-25292
- PubMed: 26296892
- DOI: https://doi.org/10.1074/jbc.M115.637207
- Primary Citation of Related Structures:
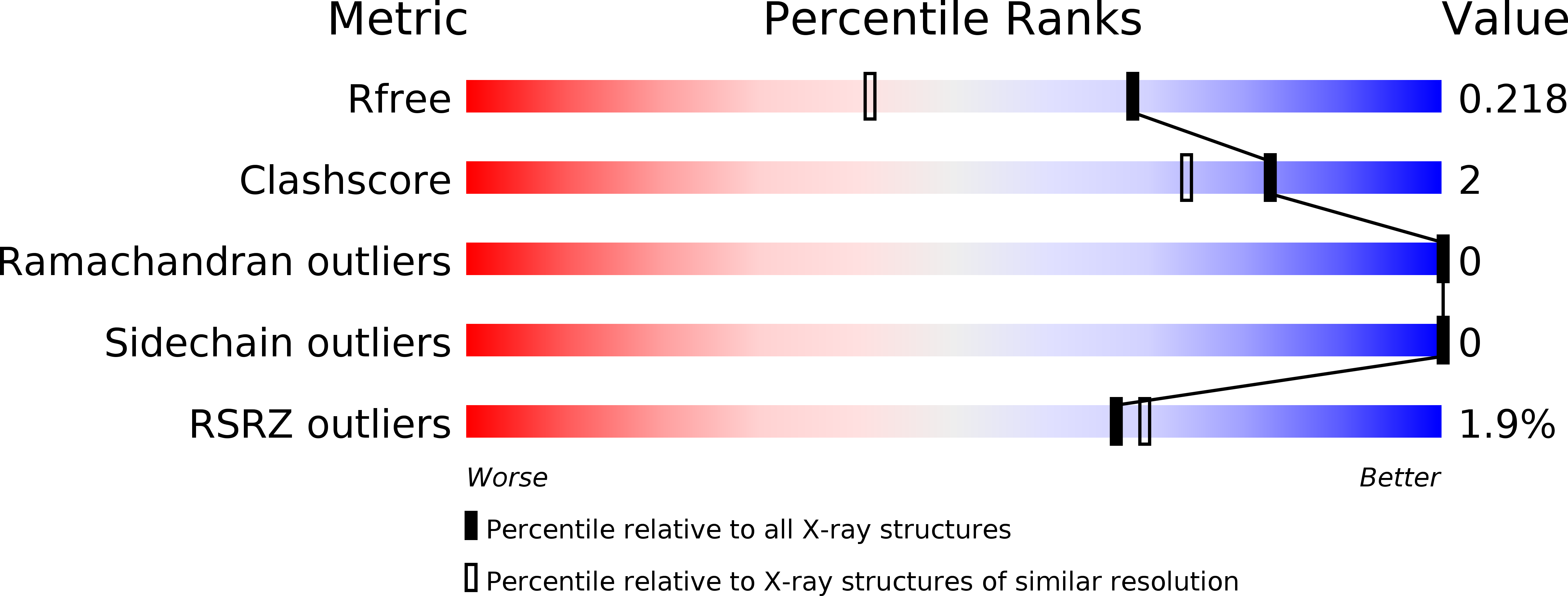
3U23, 4WCI - PubMed Abstract:
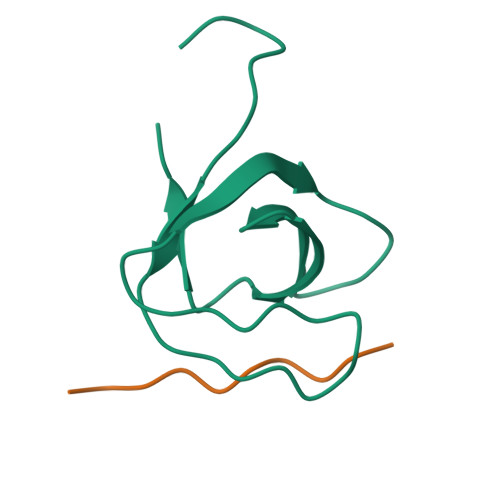
CD2AP is an adaptor protein involved in membrane trafficking, with essential roles in maintaining podocyte function within the kidney glomerulus. CD2AP contains three Src homology 3 (SH3) domains that mediate multiple protein-protein interactions. However, a detailed comparison of the molecular binding preferences of each SH3 remained unexplored, as well as the discovery of novel interactors. Thus, we studied the binding properties of each SH3 domain to the known interactor Casitas B-lineage lymphoma protein (c-CBL), conducted a peptide array screen based on the recognition motif PxPxPR and identified 40 known or novel candidate binding proteins, such as RIN3, a RAB5-activating guanine nucleotide exchange factor. CD2AP SH3 domains 1 and 2 generally bound with similar characteristics and specificities, whereas the SH3-3 domain bound more weakly to most peptide ligands tested yet recognized an unusually extended sequence in ALG-2-interacting protein X (ALIX). RIN3 peptide scanning arrays revealed two CD2AP binding sites, recognized by all three SH3 domains, but SH3-3 appeared non-functional in precipitation experiments. RIN3 recruited CD2AP to RAB5a-positive early endosomes via these interaction sites. Permutation arrays and isothermal titration calorimetry data showed that the preferred binding motif is Px(P/A)xPR. Two high-resolution crystal structures (1.65 and 1.11 Å) of CD2AP SH3-1 and SH3-2 solved in complex with RIN3 epitopes 1 and 2, respectively, indicated that another extended motif is relevant in epitope 2. In conclusion, we have discovered novel interaction candidates for CD2AP and characterized subtle yet significant differences in the recognition preferences of its three SH3 domains for c-CBL, ALIX, and RIN3.
Organizational Affiliation:
From the Weatherall Institute of Molecular Medicine, Department of Oncology, University of Oxford, Oxford OX3 9DS, United Kingdom.