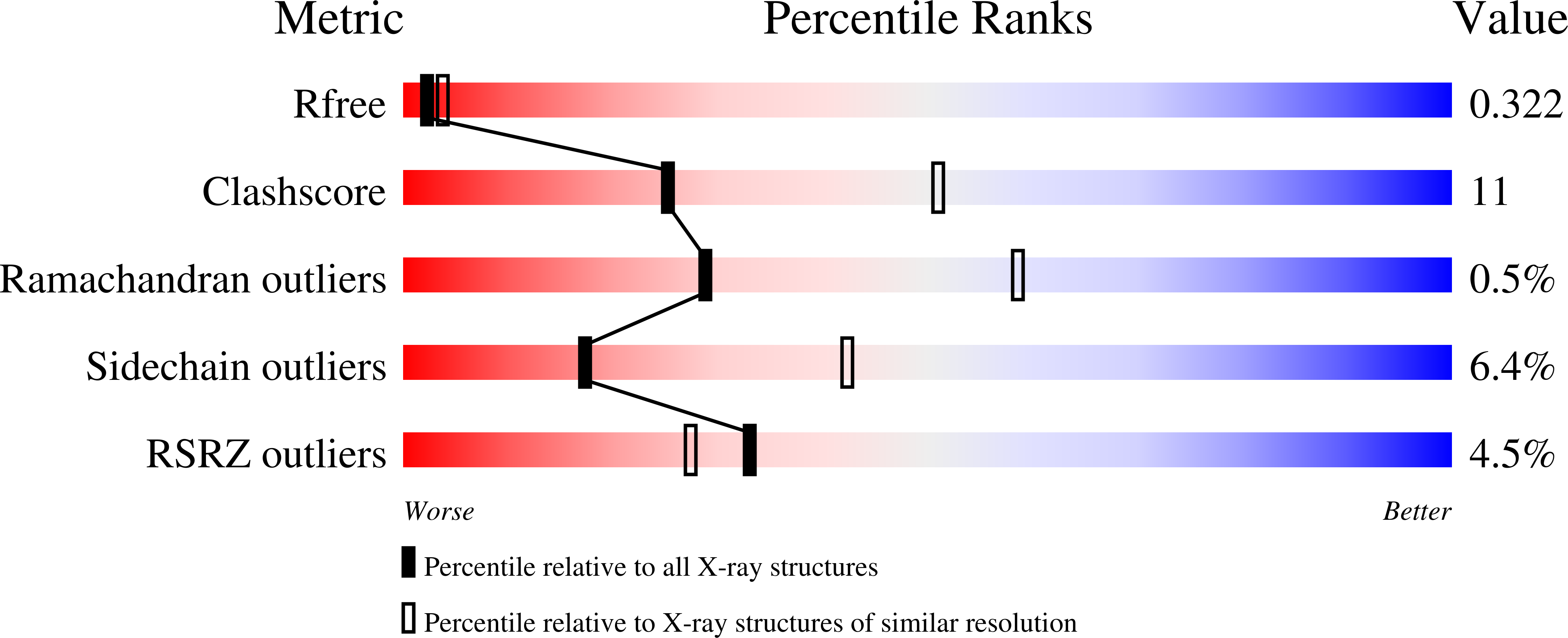
Structure of the full-length insecticidal protein Cry1Ac reveals intriguing details of toxin packaging into in vivo formed crystals.
Evdokimov, A.G., Moshiri, F., Sturman, E.J., Rydel, T.J., Zheng, M., Seale, J.W., Franklin, S.(2014) Protein Sci 23: 1491-1497
- PubMed: 25139047
- DOI: https://doi.org/10.1002/pro.2536
- Primary Citation of Related Structures:
4W8J - PubMed Abstract:
For almost half a century, the structure of the full-length Bacillus thuringiensis (Bt) insecticidal protein Cry1Ac has eluded researchers, since Bt-derived crystals were first characterized in 1965. Having finally solved this structure we report intriguing details of the lattice-based interactions between the toxic core of the protein and the protoxin domains. The structure provides concrete evidence for the function of the protoxin as an enhancer of native crystal packing and stability.
Organizational Affiliation:
Monsanto, GG4D 700 Chesterfield Parkway West, Chesterfield, Missouri, 63017.