Interactions by the Fungal Flo11 Adhesin Depend on a Fibronectin Type III-Like Adhesin Domain Girdled by Aromatic Bands.
Kraushaar, T., Bruckner, S., Veelders, M., Rhinow, D., Schreiner, F., Birke, R., Pagenstecher, A., Mosch, H., Essen, L.(2015) Structure 23: 1005
- PubMed: 25960408
- DOI: https://doi.org/10.1016/j.str.2015.03.021
- Primary Citation of Related Structures:
4UYR, 4UYS, 4UYT - PubMed Abstract:
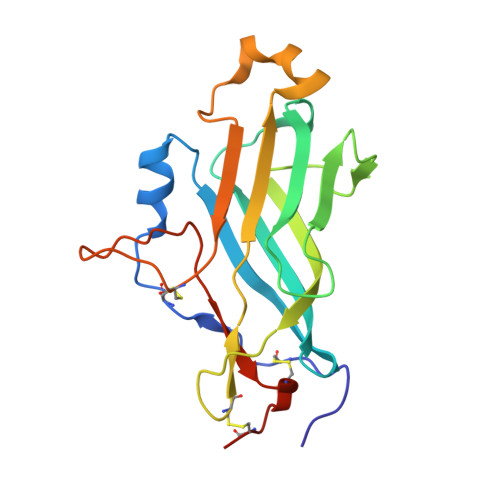
Saccharomyces cerevisiae harbors a family of GPI-anchored cell wall proteins for interaction with its environment. The flocculin Flo11, a major representative of these fungal adhesins, confers formation of different types of multicellular structures such as biofilms, flors, or filaments. To understand these environment-dependent growth phenotypes on a molecular level, we solved the crystal structure of the N-terminal Flo11A domain at 0.89-Å resolution. Besides a hydrophobic apical region, the Flo11A domain consists of a β sandwich of the fibronectin type III domain (FN3). We further show that homophilic Flo11-Flo11 interactions and heterophilic Flo11-plastic interactions solely depend on the Flo11A domain and are strongly pH dependent. These functions of Flo11A involve an apical region with its surface-exposed aromatic band, which is accompanied by acidic stretches. Together with electron microscopic reconstructions of yeast cell-cell contact sites, our data suggest that Flo11 acts as a spacer-like, pH-sensitive adhesin that resembles a membrane-tethered hydrophobin.
Organizational Affiliation:
Department of Chemistry, Philipps-Universität, Hans-Meerwein-Straße 4, 35032 Marburg, Germany; Department of Biology, Philipps-Universität, Karl-von-Frisch-Straße 8, 35043 Marburg, Germany.