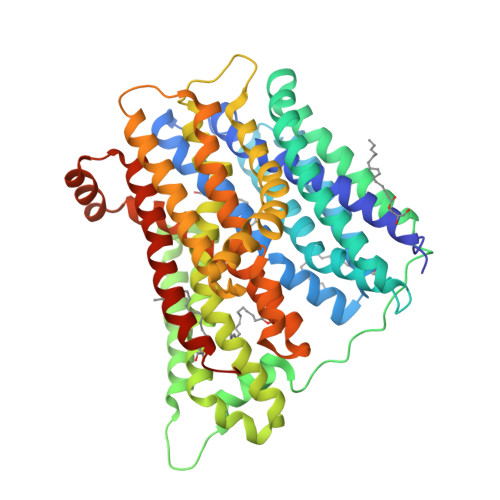
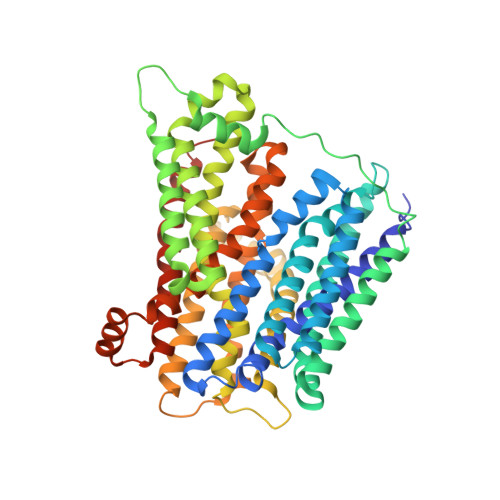
Gating Topology of the Proton-Coupled Oligopeptide Symporters.
Fowler, P.W., Orwick-Rydmark, M., Radestock, S., Solcan, N., Dijkman, P.M., Lyons, J.A., Kwok, J., Caffrey, M., Watts, A., Forrest, L.R., Newstead, S.(2015) Structure 23: 290
- PubMed: 25651061
- DOI: https://doi.org/10.1016/j.str.2014.12.012
- Primary Citation of Related Structures:
4UVM - PubMed Abstract:
Proton-coupled oligopeptide transporters belong to the major facilitator superfamily (MFS) of membrane transporters. Recent crystal structures suggest the MFS fold facilitates transport through rearrangement of their two six-helix bundles around a central ligand binding site; how this is achieved, however, is poorly understood. Using modeling, molecular dynamics, crystallography, functional assays, and site-directed spin labeling combined with double electron-electron resonance (DEER) spectroscopy, we present a detailed study of the transport dynamics of two bacterial oligopeptide transporters, PepTSo and PepTSt. Our results identify several salt bridges that stabilize outward-facing conformations and we show that, for all the current structures of MFS transporters, the first two helices of each of the four inverted-topology repeat units form half of either the periplasmic or cytoplasmic gate and that these function cooperatively in a scissor-like motion to control access to the peptide binding site during transport.
Organizational Affiliation:
Department of Biochemistry, University of Oxford, South Parks Road, Oxford OX1 3QU, UK. Electronic address: philip.fowler@bioch.ox.ac.uk.