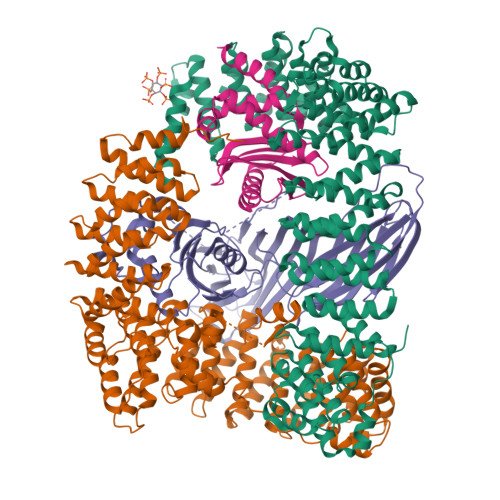
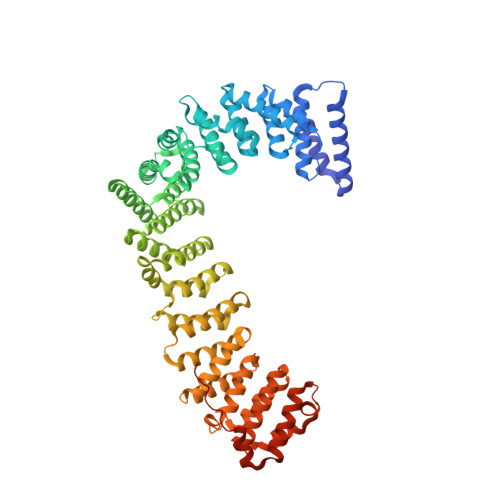
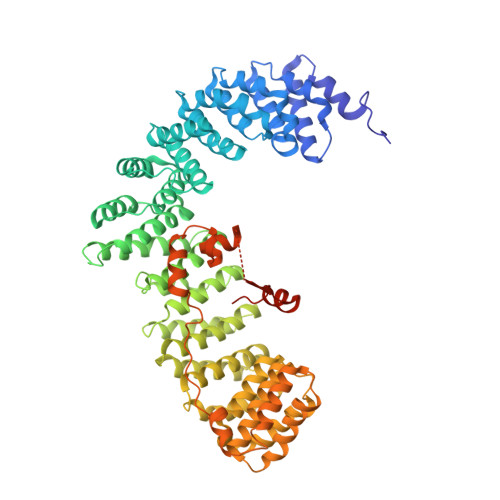
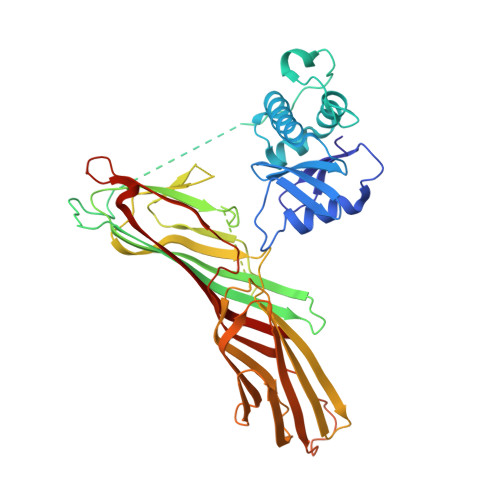
Clathrin Adaptors. Ap2 Controls Clathrin Polymerization with a Membrane-Activated Switch.
Kelly, B.T., Graham, S.C., Liska, N., Dannhauser, P.N., Honing, S., Ungewickell, E.J., Owen, D.J.(2014) Science 345: 459
- PubMed: 25061211
- DOI: https://doi.org/10.1126/science.1254836
- Primary Citation of Related Structures:
4UQI - PubMed Abstract:
Clathrin-mediated endocytosis (CME) is vital for the internalization of most cell-surface proteins. In CME, plasma membrane-binding clathrin adaptors recruit and polymerize clathrin to form clathrin-coated pits into which cargo is sorted. Assembly polypeptide 2 (AP2) is the most abundant adaptor and is pivotal to CME. Here, we determined a structure of AP2 that includes the clathrin-binding β2 hinge and developed an AP2-dependent budding assay. Our findings suggest that an autoinhibitory mechanism prevents clathrin recruitment by cytosolic AP2. A large-scale conformational change driven by the plasma membrane phosphoinositide phosphatidylinositol 4,5-bisphosphate and cargo relieves this autoinhibition, triggering clathrin recruitment and hence clathrin-coated bud formation. This molecular switching mechanism can couple AP2's membrane recruitment to its key functions of cargo and clathrin binding.
Organizational Affiliation:
Cambridge Institute for Medical Research (CIMR), Department of Clinical Biochemistry, University of Cambridge, Hills Road, Cambridge CB2 0XY, UK. btk1000@cam.ac.uk djo30@cam.ac.uk.