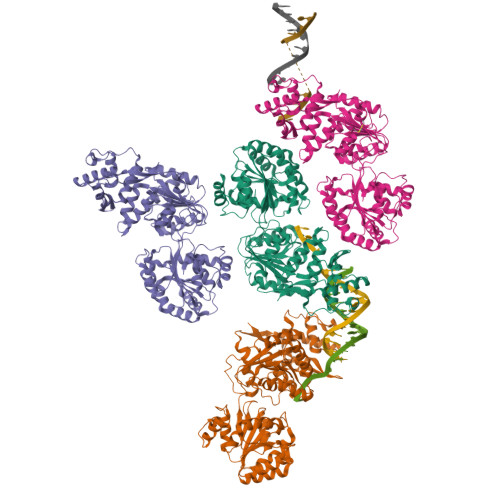
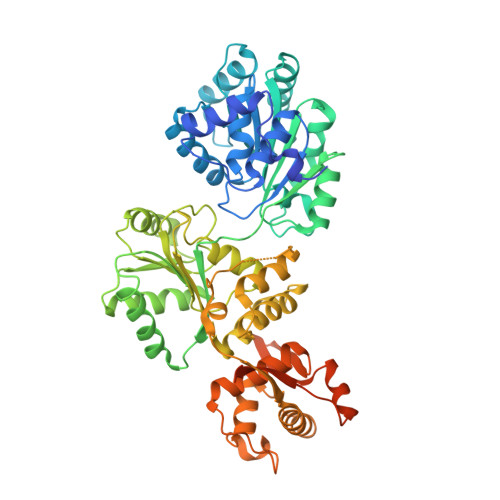
Human RECQ1 helicase-driven DNA unwinding, annealing, and branch migration: Insights from DNA complex structures.
Pike, A.C., Gomathinayagam, S., Swuec, P., Berti, M., Zhang, Y., Schnecke, C., Marino, F., von Delft, F., Renault, L., Costa, A., Gileadi, O., Vindigni, A.(2015) Proc Natl Acad Sci U S A 112: 4286-4291
- PubMed: 25831490
- DOI: https://doi.org/10.1073/pnas.1417594112
- Primary Citation of Related Structures:
2WWY, 4U7D - PubMed Abstract:
RecQ helicases are a widely conserved family of ATP-dependent motors with diverse roles in nearly every aspect of bacterial and eukaryotic genome maintenance. However, the physical mechanisms by which RecQ helicases recognize and process specific DNA replication and repair intermediates are largely unknown. Here, we solved crystal structures of the human RECQ1 helicase in complexes with tailed-duplex DNA and ssDNA. The structures map the interactions of the ssDNA tail and the branch point along the helicase and Zn-binding domains, which, together with reported structures of other helicases, define the catalytic stages of helicase action. We also identify a strand-separating pin, which (uniquely in RECQ1) is buttressed by the protein dimer interface. A duplex DNA-binding surface on the C-terminal domain is shown to play a role in DNA unwinding, strand annealing, and Holliday junction (HJ) branch migration. We have combined EM and analytical ultracentrifugation approaches to show that RECQ1 can form what appears to be a flat, homotetrameric complex and propose that RECQ1 tetramers are involved in HJ recognition. This tetrameric arrangement suggests a platform for coordinated activity at the advancing and receding duplexes of an HJ during branch migration.
Organizational Affiliation:
Structural Genomics Consortium, University of Oxford, Oxford OX3 7DQ, United Kingdom;