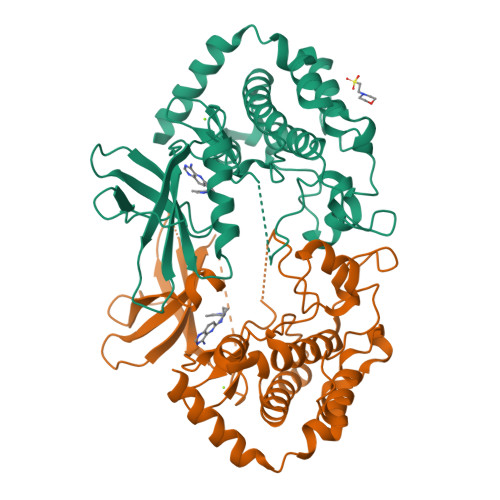
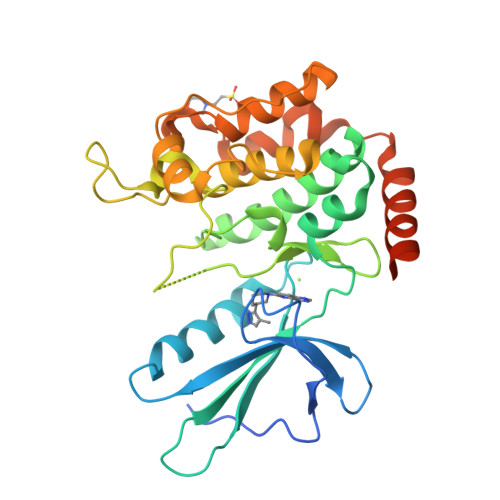
Structural Plasticity and Kinase Activation in a Cohort of MAP4K4 Structures
Wu, P., Chen, H., Coons, M., Crawford, T.D., Kirkpatrick, D.S., Murray, L., Ndubaku, C.O., Nonomiya, J., Pham, V., Schmidt, S., Smysczek, T., Vitorino, P., Ye, W., Harris, S.F.To be published.