Structural and Biochemical Studies of a Moderately Thermophilic Exonuclease I from Methylocaldum szegediense.
Fei, L., Tian, S., Moysey, R., Misca, M., Barker, J.J., Smith, M.A., McEwan, P.A., Pilka, E.S., Crawley, L., Evans, T., Sun, D.(2015) PLoS One 10: e0117470-e0117470
- PubMed: 25658953
- DOI: https://doi.org/10.1371/journal.pone.0117470
- Primary Citation of Related Structures:
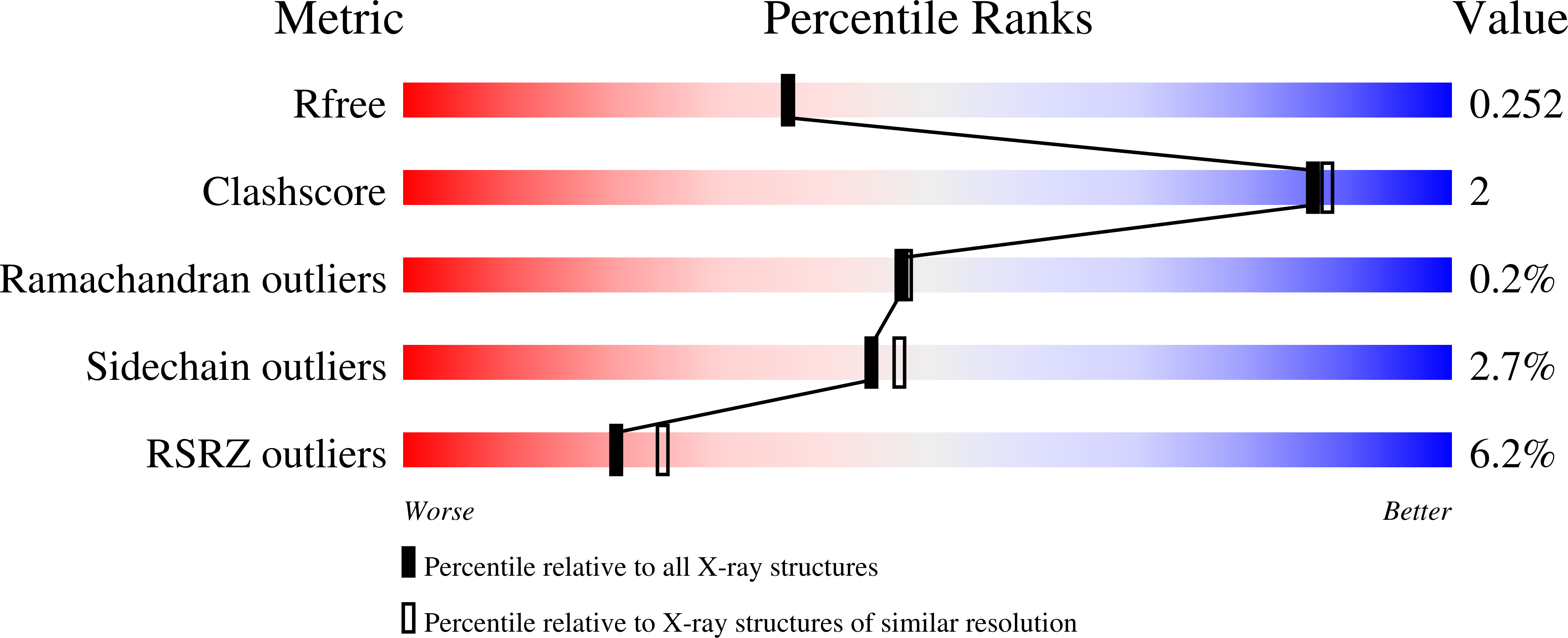
4RG8 - PubMed Abstract:
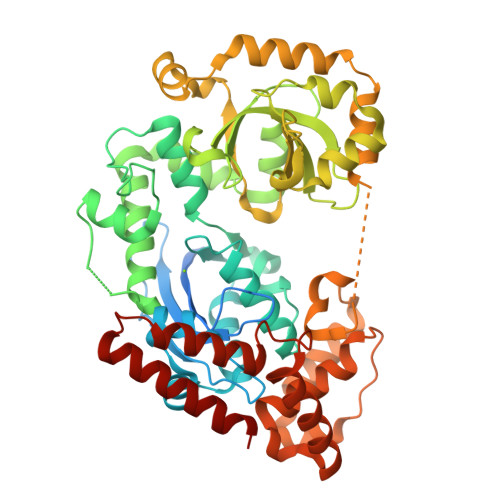
A novel exonuclease, designated as MszExo I, was cloned from Methylocaldum szegediense, a moderately thermophilic methanotroph. It specifically digests single-stranded DNA in the 3' to 5' direction. The protein is composed of 479 amino acids, and it shares 47% sequence identity with E. coli Exo I. The crystal structure of MszExo I was determined to a resolution of 2.2 Å and it aligns well with that of E. coli Exo I. Comparative studies revealed that MszExo I and E. coli Exo I have similar metal ion binding affinity and similar activity at mesophilic temperatures (25-47°C). However, the optimum working temperature of MszExo I is 10°C higher, and the melting temperature is more than 4°C higher as evaluated by both thermal inactivation assays and DSC measurements. More importantly, two thermal transitions during unfolding of MszExo I were monitored by DSC while only one transition was found in E. coli Exo I. Further analyses showed that magnesium ions not only confer structural stability, but also affect the unfolding of MszExo I. MszExo I is the first reported enzyme in the DNA repair systems of moderately thermophilic bacteria, which are predicted to have more efficient DNA repair systems than mesophilic ones.
Organizational Affiliation:
New England Biolabs Shanghai R&D Center, Building 5, 917 Halei Road, Pudong District, Shanghai, China.