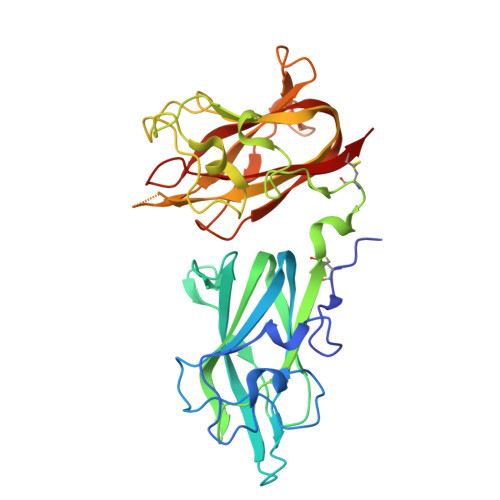
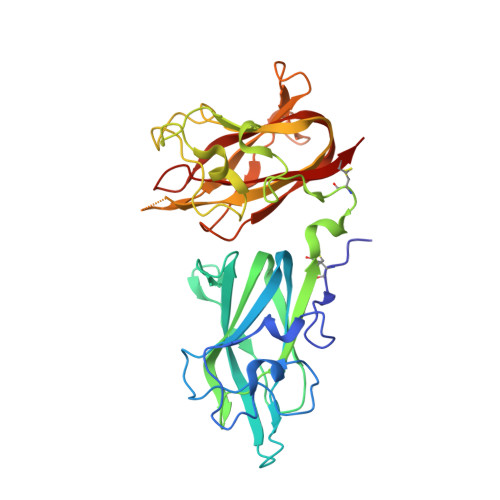
Structural Basis for VEGF-C Binding to Neuropilin-2 and Sequestration by a Soluble Splice Form.
Parker, M.W., Linkugel, A.D., Goel, H.L., Wu, T., Mercurio, A.M., Vander Kooi, C.W.(2015) Structure 23: 677-687
- PubMed: 25752543
- DOI: https://doi.org/10.1016/j.str.2015.01.018
- Primary Citation of Related Structures:
4QDQ, 4QDR, 4QDS - PubMed Abstract:
Vascular endothelial growth factor C (VEGF-C) is a potent lymphangiogenic cytokine that signals via the coordinated action of two cell surface receptors, Neuropilin-2 (Nrp2) and VEGFR-3. Diseases associated with both loss and gain of VEGF-C function, lymphedema and cancer, respectively, motivate studies of VEGF-C/Nrp2 binding and inhibition. Here, we demonstrate that VEGF-C binding to Nrp2 is regulated by C-terminal proteolytic maturation. The structure of the VEGF-C C terminus in complex with the ligand binding domains of Nrp2 demonstrates that a cryptic Nrp2 binding motif is released upon proteolysis, allowing specific engagement with the b1 domain of Nrp2. Based on the identified structural requirements for Nrp2 binding to VEGF-C, we hypothesized that the endogenous secreted splice form of Nrp2, s9Nrp2, may function as a selective inhibitor of VEGF-C. We find that s9Nrp2 forms a stable dimer that potently inhibits VEGF-C/Nrp2 binding and cellular signaling. These data provide critical insight into VEGF-C/Nrp2 binding and inhibition.
Organizational Affiliation:
Department of Molecular and Cellular Biochemistry, Center for Structural Biology, University of Kentucky, Lexington, KY 40536, USA.