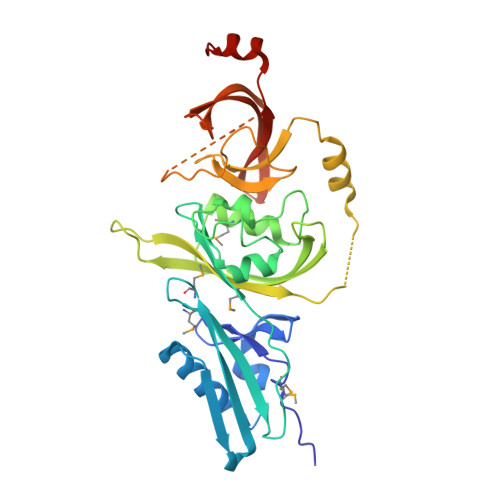
Mycobacterium tuberculosis Rv3651 is a triple sensor-domain protein.
Abendroth, J., Frando, A., Phan, I.Q., Staker, B.L., Myler, P.J., Edwards, T.E., Grundner, C.(2018) Protein Sci 27: 568-572
- PubMed: 29119630
- DOI: https://doi.org/10.1002/pro.3343
- Primary Citation of Related Structures:
4Q6U - PubMed Abstract:
The genome of the human pathogen Mycobacterium tuberculosis (Mtb) encodes ∼4,400 proteins, but one third of them have unknown functions. We solved the crystal structure of Rv3651, a hypothetical protein with no discernible similarity to proteins with known function. Rv3651 has a three-domain architecture that combines one cGMP-specific phosphodiesterases, adenylyl cyclases and FhlA (GAF) domain and two Per-ARNT-Sim (PAS) domains. GAF and PAS domains are sensor domains that are typically linked to signaling effector molecules. Unlike these sensor-effector proteins, Rv3651 is an unusual sensor domain-only protein with highly divergent sequence. The structure suggests that Rv3651 integrates multiple different signals and serves as a scaffold to facilitate signal transfer.
Organizational Affiliation:
Beryllium Discovery, Bainbridge Island, Washington.