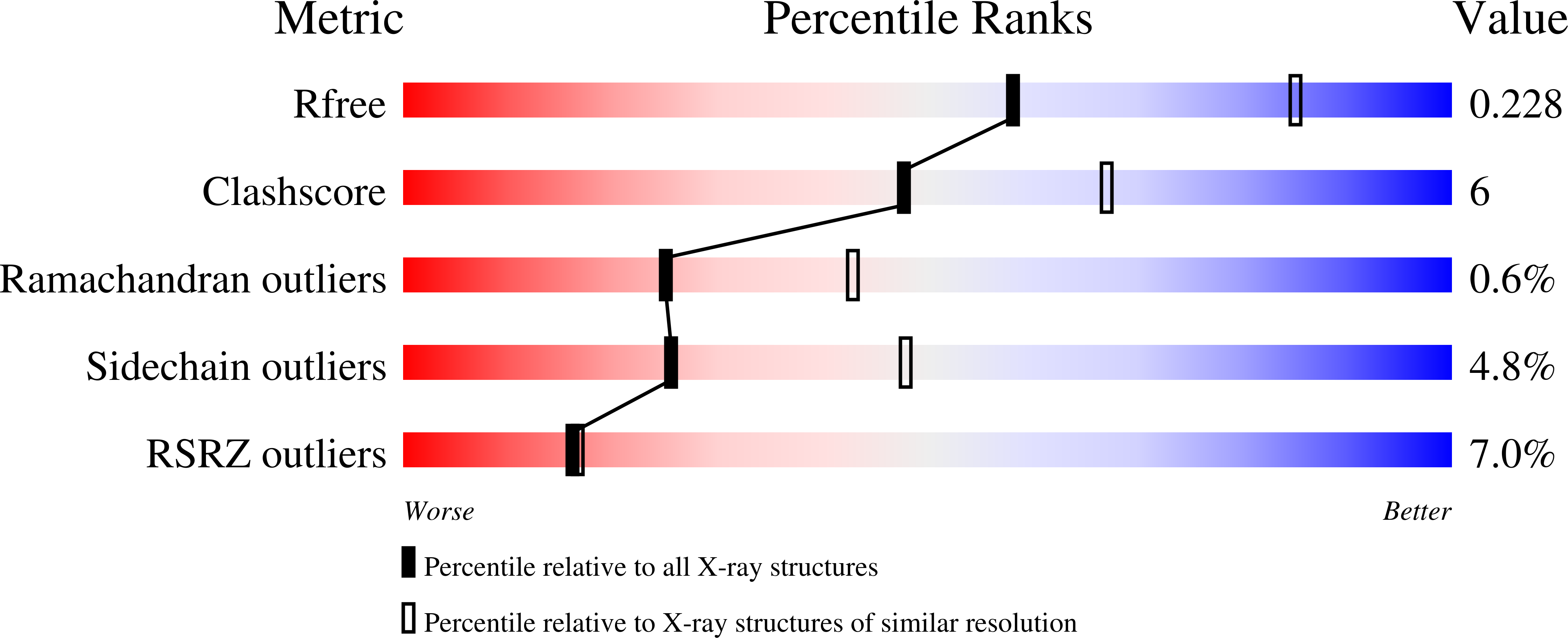
Distinctive Properties of the Nuclear Localization Signals of Inner Nuclear Membrane Proteins Heh1 and Heh2.
Lokareddy, R.K., Hapsari, R.A., van Rheenen, M., Pumroy, R.A., Bhardwaj, A., Steen, A., Veenhoff, L.M., Cingolani, G.(2015) Structure 23: 1305-1316
- PubMed: 26051712
- DOI: https://doi.org/10.1016/j.str.2015.04.017
- Primary Citation of Related Structures:
4PVZ, 4XZR - PubMed Abstract:
Targeting of ER-synthesized membrane proteins to the inner nuclear membrane (INM) has long been explained by the diffusion-retention model. However, several INM proteins contain non-classical nuclear localization signal (NLS) sequences, which, in a few instances, have been shown to promote importin α/β- and Ran-dependent translocation to the INM. Here, using structural and biochemical methods, we show that yeast INM proteins Heh2 and Src1/Heh1 contain bipartite import sequences that associate intimately with the minor NLS-binding pocket of yeast importin α and unlike classical NLSs efficiently displace the IBB domain in the absence of importin β. In vivo, the intimate interactions at the minor NLS-binding pocket make the h2NLS highly efficient at recruiting importin α at the ER and drive INM localization of endogenous Heh2. Thus, h1/h2NLSs delineate a novel class of super-potent, IBB-like membrane protein NLSs, distinct from classical NLSs found in soluble cargos and of general interest in biology.
Organizational Affiliation:
Dept. of Biochemistry and Molecular Biology, Thomas Jefferson University, 233 South 10 Street, Philadelphia, PA 19107, USA.