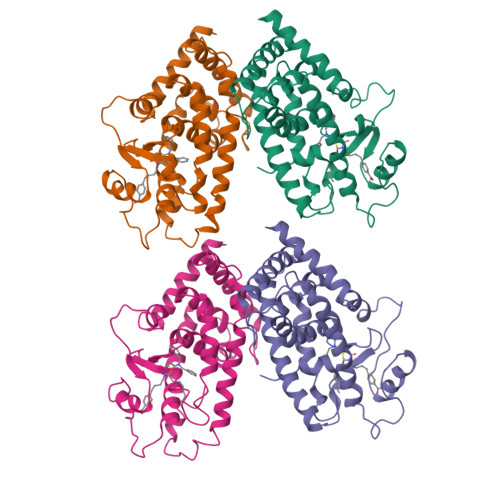
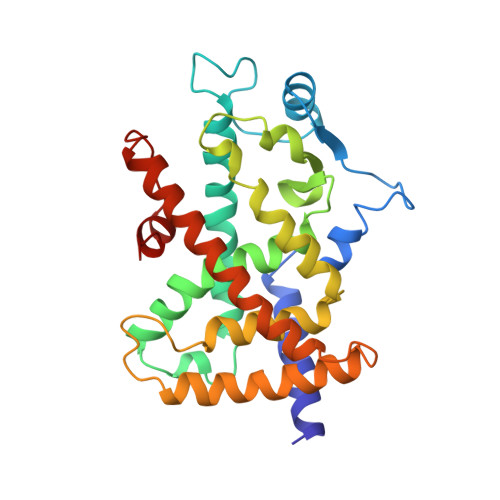
A peroxisome proliferator-activated receptor gamma ligand inhibits adipocyte differentiation.
Oberfield, J.L., Collins, J.L., Holmes, C.P., Goreham, D.M., Cooper, J.P., Cobb, J.E., Lenhard, J.M., Hull-Ryde, E.A., Mohr, C.P., Blanchard, S.G., Parks, D.J., Moore, L.B., Lehmann, J.M., Plunket, K., Miller, A.B., Milburn, M.V., Kliewer, S.A., Willson, T.M.(1999) Proc Natl Acad Sci U S A 96: 6102-6106
- PubMed: 10339548
- DOI: https://doi.org/10.1073/pnas.96.11.6102
- Primary Citation of Related Structures:
4PRG - PubMed Abstract:
The peroxisome proliferator-activated receptors (PPARs) are nuclear hormone receptors that regulate glucose and lipid homeostasis. The PPARgamma subtype plays a central role in the regulation of adipogenesis and is the molecular target for the 2, 4-thiazolidinedione class of antidiabetic drugs. Structural studies have revealed that agonist ligands activate the PPARs through direct interactions with the C-terminal region of the ligand-binding domain, which includes the activation function 2 helix. GW0072 was identified as a high-affinity PPARgamma ligand that was a weak partial agonist of PPARgamma transactivation. X-ray crystallography revealed that GW0072 occupied the ligand-binding pocket by using different epitopes than the known PPAR agonists and did not interact with the activation function 2 helix. In cell culture, GW0072 was a potent antagonist of adipocyte differentiation. These results establish an approach to the design of PPAR ligands with modified biological activities.
Organizational Affiliation:
Department of Molecular Endocrinology, Glaxo Wellcome Research and Development, Research Triangle Park, NC 27709, USA.