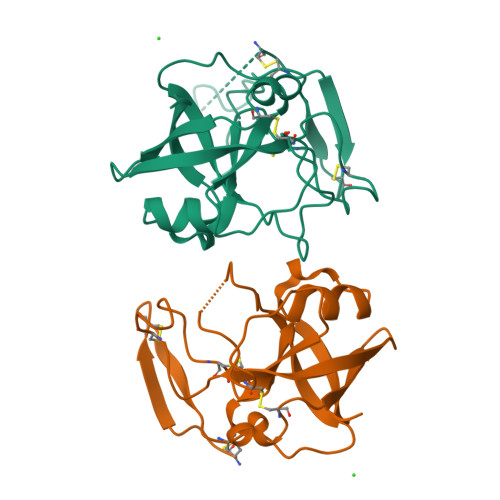
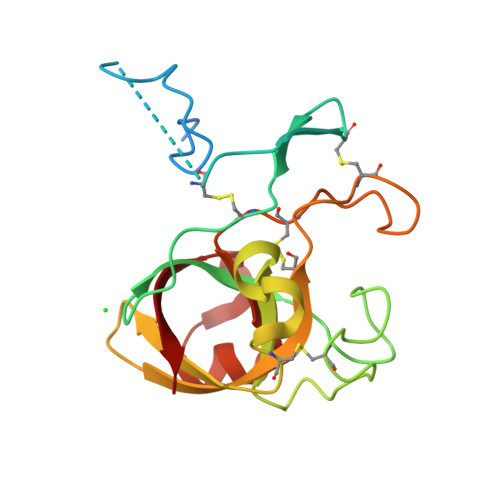
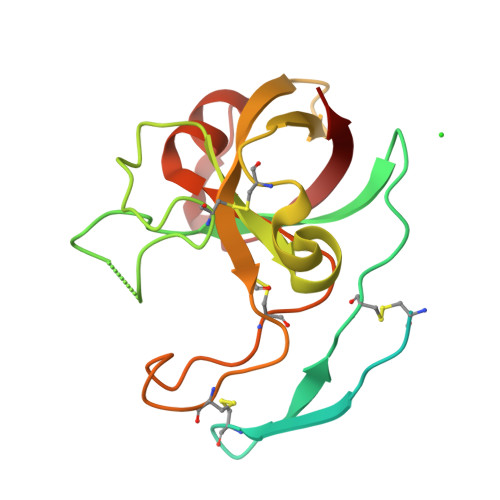
Crystal structure of kiwellin, a major cell-wall protein from kiwifruit.
Hamiaux, C., Maddumage, R., Middleditch, M.J., Prakash, R., Brummell, D.A., Baker, E.N., Atkinson, R.G.(2014) J Struct Biol 187: 276-281
- PubMed: 25093947
- DOI: https://doi.org/10.1016/j.jsb.2014.07.005
- Primary Citation of Related Structures:
4PMK - PubMed Abstract:
Kiwellin is a cysteine-rich, cell wall-associated protein with no known structural homologues. It is one of the most abundant proteins in kiwifruit (Actinidia spp.), and has been shown to be recognised by IgE of some patients allergic to kiwifruit. Cleavage of kiwellin into an N-terminal 4 kDa peptide called kissper and a core domain called KiTH is mediated by actinidin in vitro, and isolation of the kissper peptide from green-fleshed kiwifruit extracts suggested it may result from in vivo processing of kiwellin. In solution, kissper is highly flexible and displays pore-forming activity in synthetic lipid-bilayers. We present here the 2.05 Å resolution crystal structure of full-length kiwellin, purified from its native source, Actinidia chinensis (gold-fleshed kiwifruit). The structure confirms the modularity of the protein and the intrinsic flexibility of kissper and reveals that KiTH harbours a double-psi β-barrel fold hooked to an N-terminal β hairpin. Comparisons with structurally-related proteins suggest that a deep gorge located at the protein surface forms a binding site for endogenous ligands.
Organizational Affiliation:
The New Zealand Institute for Plant & Food Research Limited, Private Bag 92169, Auckland 1142, New Zealand. Electronic address: cyril.hamiaux@plantandfood.co.nz.