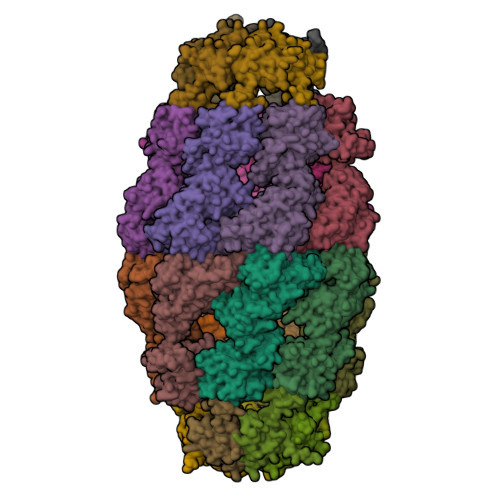
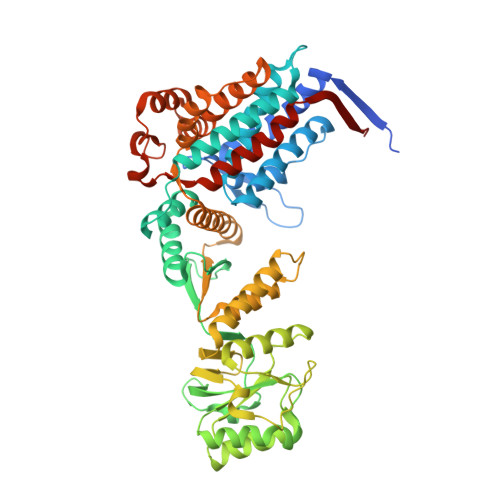
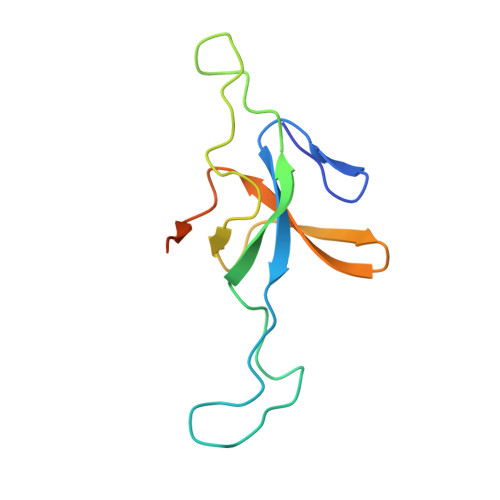
Crystal structure of the human mitochondrial chaperonin symmetrical football complex.
Nisemblat, S., Yaniv, O., Parnas, A., Frolow, F., Azem, A.(2015) Proc Natl Acad Sci U S A 112: 6044-6049
- PubMed: 25918392
- DOI: https://doi.org/10.1073/pnas.1411718112
- Primary Citation of Related Structures:
4PJ1 - PubMed Abstract:
Human mitochondria harbor a single type I chaperonin system that is generally thought to function via a unique single-ring intermediate. To date, no crystal structure has been published for any mammalian type I chaperonin complex. In this study, we describe the crystal structure of a football-shaped, double-ring human mitochondrial chaperonin complex at 3.15 Å, which is a novel intermediate, likely representing the complex in an early stage of dissociation. Interestingly, the mitochondrial chaperonin was captured in a state that exhibits subunit asymmetry within the rings and nucleotide symmetry between the rings. Moreover, the chaperonin tetradecamers show a different interring subunit arrangement when compared to GroEL. Our findings suggest that the mitochondrial chaperonins use a mechanism that is distinct from the mechanism of the well-studied Escherichia coli system.
Organizational Affiliation:
Departments of Biochemistry and Molecular Biology and The Daniella Rich Institute for Structural Biology, The George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv 69978, Israel.