Fully Human VH Single Domains That Rival the Stability and Cleft Recognition of Camelid Antibodies.
Rouet, R., Dudgeon, K., Christie, M., Langley, D., Christ, D.(2015) J Biological Chem 290: 11905-11917
- PubMed: 25737448
- DOI: https://doi.org/10.1074/jbc.M114.614842
- Primary Citation of Related Structures:
4PGJ, 4U3X - PubMed Abstract:
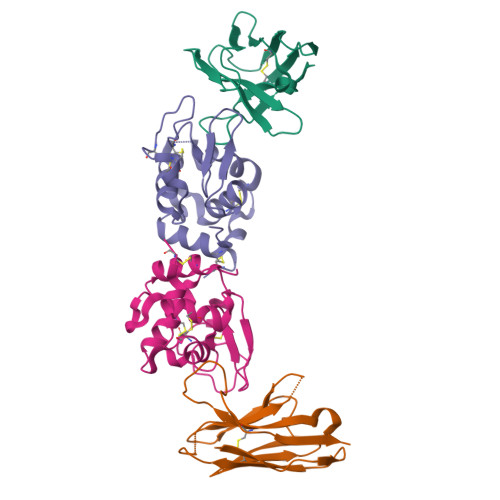
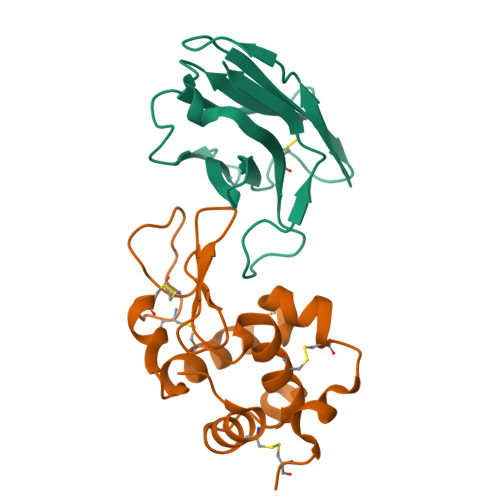
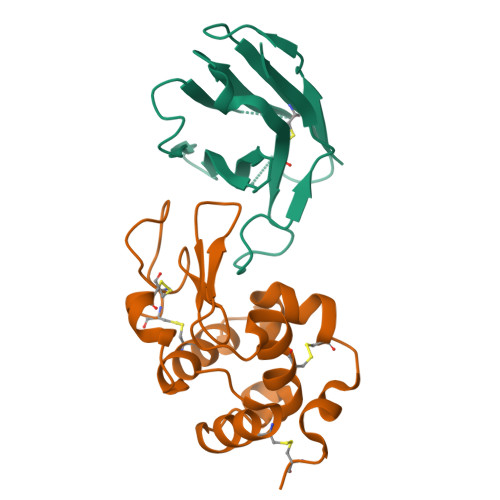
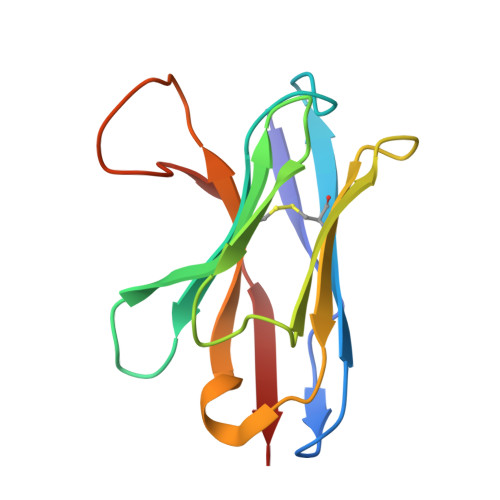
Human VH single domains represent a promising class of antibody fragments with applications as therapeutic modalities. Unfortunately, isolated human VH domains also generally display poor biophysical properties and a propensity to aggregate. This has encouraged the development of non-human antibody domains as alternative means of antigen recognition and, in particular, camelid (VHH) domains. Naturally devoid of light chain partners, these domains are characterized by favorable biophysical properties and propensity for cleft binding, a highly desirable characteristic, allowing the targeting of cryptic epitopes. In contrast, previously reported structures of human VH single domains had failed to recapitulate this property. Here we report the engineering and characterization of phage display libraries of stable human VH domains and the selection of binders against a diverse set of antigens. Unlike "camelized" human domains, the domains do not rely on potentially immunogenic framework mutations and maintain the structure of the VH/VL interface. Structure determination in complex with hen egg white lysozyme revealed an extended VH binding interface, with complementarity-determining region 3 deeply penetrating into the active site cleft, highly reminiscent of what has been observed for camelid domains. Taken together, our results demonstrate that fully human VH domains can be constructed that are not only stable and well expressed but also rival the cleft binding properties of camelid antibodies.
Organizational Affiliation:
From the Department of Immunology, Garvan Institute of Medical Research, 384 Victoria Street, Darlinghurst, Sydney, New South Wales 2010, Australia and.