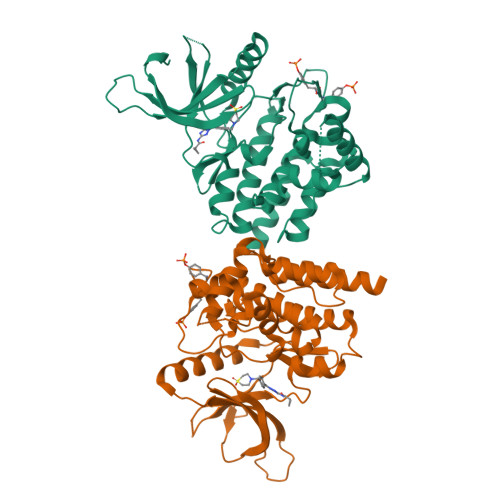
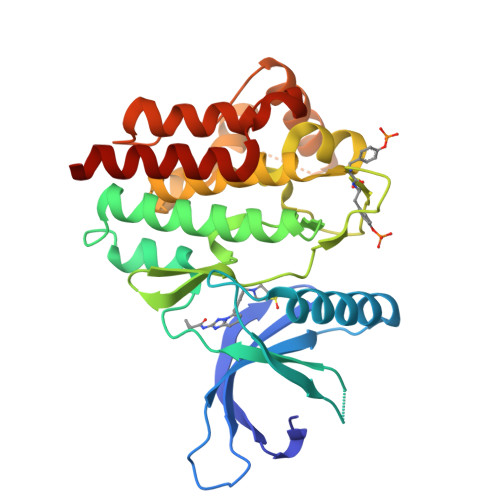
Triazolopyridines as Selective JAK1 Inhibitors: From Hit Identification to GLPG0634.
Menet, C.J., Fletcher, S.R., Van Lommen, G., Geney, R., Blanc, J., Smits, K., Jouannigot, N., Deprez, P., van der Aar, E.M., Clement-Lacroix, P., Lepescheux, L., Galien, R., Vayssiere, B., Nelles, L., Christophe, T., Brys, R., Uhring, M., Ciesielski, F., Van Rompaey, L.(2014) J Med Chem 57: 9323-9342
- PubMed: 25369270
- DOI: https://doi.org/10.1021/jm501262q
- Primary Citation of Related Structures:
4P7E - PubMed Abstract:
Janus kinases (JAK1, JAK2, JAK3, and TYK2) are involved in the signaling of multiple cytokines important in cellular function. Blockade of the JAK-STAT pathway with a small molecule has been shown to provide therapeutic immunomodulation. Having identified JAK1 as a possible new target for arthritis at Galapagos, the compound library was screened against JAK1, resulting in the identification of a triazolopyridine-based series of inhibitors represented by 3. Optimization within this chemical series led to identification of GLPG0634 (65, filgotinib), a selective JAK1 inhibitor currently in phase 2B development for RA and phase 2A development for Crohn's disease (CD).
Organizational Affiliation:
Galapagos NV , Generaal de Wittelaan L11A3, 2800 Mechelen, Belgium.