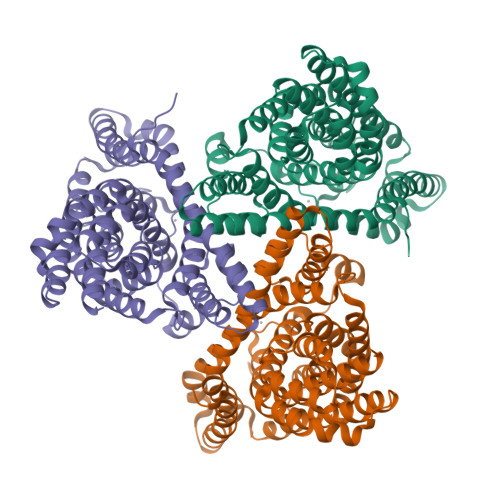
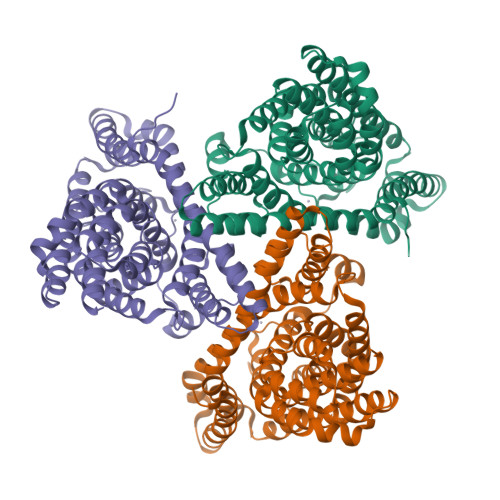
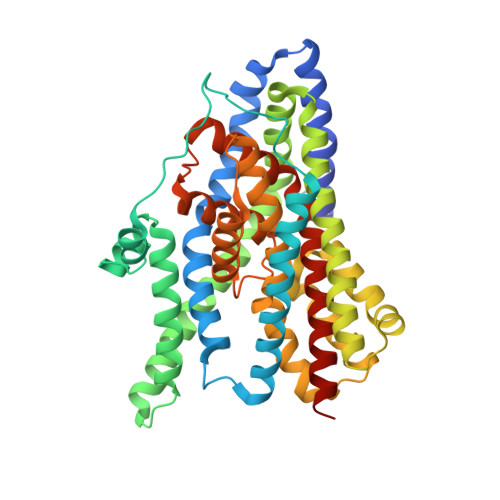
Coupled ion binding and structural transitions along the transport cycle of glutamate transporters.
Verdon, G., Oh, S., Serio, R.N., Boudker, O.(2014) Elife 3: e02283-e02283
- PubMed: 24842876
- DOI: https://doi.org/10.7554/eLife.02283
- Primary Citation of Related Structures:
4OYE, 4OYF, 4P19, 4P1A, 4P3J, 4P6H, 5CFY - PubMed Abstract:
Membrane transporters that clear the neurotransmitter glutamate from synapses are driven by symport of sodium ions and counter-transport of a potassium ion. Previous crystal structures of a homologous archaeal sodium and aspartate symporter showed that a dedicated transport domain carries the substrate and ions across the membrane. Here, we report new crystal structures of this homologue in ligand-free and ions-only bound outward- and inward-facing conformations. We show that after ligand release, the apo transport domain adopts a compact and occluded conformation that can traverse the membrane, completing the transport cycle. Sodium binding primes the transport domain to accept its substrate and triggers extracellular gate opening, which prevents inward domain translocation until substrate binding takes place. Furthermore, we describe a new cation-binding site ideally suited to bind a counter-transported ion. We suggest that potassium binding at this site stabilizes the translocation-competent conformation of the unloaded transport domain in mammalian homologues.DOI: http://dx.doi.org/10.7554/eLife.02283.001.
Organizational Affiliation:
Department of Physiology and Biophysics, Weill Cornell Medical College, New York, United States g.verdon@imperial.ac.uk.